JEE Advance - Chemistry (1993 - No. 2)
Explanation
The reaction is $$\to$$
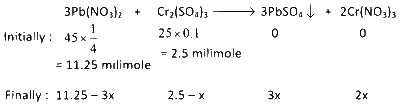
$$C{r_2}{(S{O_4})_3}$$ is limiting reagent as $${{2.5} \over 1} < {{11.25} \over 3}$$.
$$\therefore$$ $$2.5 - x = 0$$
$$ \Rightarrow x = 2.5$$ milimoles
$$\therefore$$ Moles of $$PbS{O_4}$$ formed $$ = 3x = 3 \times 2.5 = 7.5 \times {10^{ - 3}}$$ moles
After $$PbS{O_4}$$ precipitate formation in the solution $$Pb{(N{O_3})_2}$$ and $$Cr{(N{O_3})_2}$$ remains.
$$\therefore$$ Milimoles of remaining $$Pb{(N{O_3})_2}$$ is $$ = 11.25 - 3x = 11.25 - 3 \times 2.5 = 3.75$$
And milimoles of remaining $$Cr{(N{O_3})_2}$$ is $$ = 2x = 2 \times 2.5 = 5$$
$$\therefore$$ Molar concentration or molarity of $$Pb{(N{O_3})_2} = {{3.75 \times {{10}^{ - 3}}} \over {{{45 + 25} \over {1000}}}} = 0.054\,M$$
And molarity of $$Cr{(N{O_3})_2} = {{5 \times {{10}^{ - 3}}} \over {{{70} \over {1000}}}} = 0.071\,M$$
Comments (0)


