JEE Advance - Chemistry (1991 - No. 9)
Explanation
To determine which molecule or ion assumes a linear structure, we need to consider the molecular geometry, which is often predicted by the VSEPR (Valence Shell Electron Pair Repulsion) theory.
Let’s analyze each option:
Option A: SnCl2
Tin(II) chloride, SnCl2, has a bent structure rather than a linear one due to the presence of a lone pair of electrons on the tin atom, which repels the bonds between Sn and Cl.
Option B: NCO-
The cyanate ion, NCO-, has a linear structure because the central atom carbon forms a double bond with nitrogen and a triple bond with oxygen, aligning all atoms in a straight line.
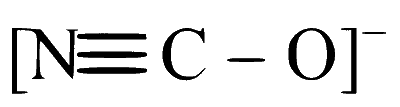
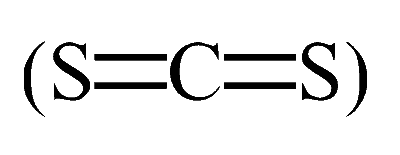
Option C: CS2
Carbon disulfide, CS2, has a linear structure. The central carbon atom forms double bonds with each sulfur atom, allowing the molecule to maintain a straight line.
Option D: $$NO_2^+$$
The nitronium ion, $$NO_2^+$$, also has a linear structure. It has a central nitrogen atom bonded to two oxygen atoms with no lone pairs on nitrogen, thus the molecule assumes a linear shape.
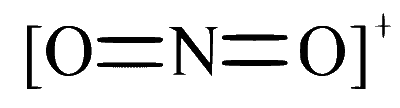
So, the linear structures are assumed by Options B, C, and D.
Comments (0)


