JEE Advance - Chemistry (1991 - No. 18)
2N2O5 (g) $$\to$$ 4NO2(g) + O2(g)
is a first order reaction. After 30 min. from the start of the decomposition in a closed vessel, the total pressure developed is found to be 284.5 mm of Hg and on complete decomposition, the total pressure is 584.5 mm of Hg. Calculate the rate constant of the reaction.
Explanation
Given reaction : 2N2O5(g) $$\to$$ 4NO2(g) + O2(g)
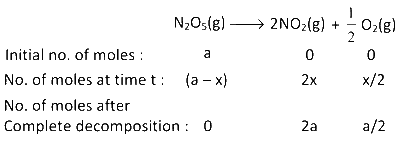
$$\therefore$$ Total no. of moles at time, $$t = a - x + 2x + {x \over 2} = a + {3 \over 2}x$$
Total no. of moles after complete decomposition
$$ = 2a + {a \over 2} = {5 \over 2}a$$
At a given volume and temperature, P $$\propto$$ n [Assuming ideal behaviour of gas mixture]
According to the given data, $${5 \over 2}$a $$\propto$$ 584.5 mm Hg ...... [1]
and $$a + {3 \over 2}x \propto 284.5$$ mm Hg ............... [2]
$$\therefore$$ a $$\propto$$ 233.8 mm Hg and $${3 \over 2}x \propto 284.5-a$$
or, $${3 \over 2}x \propto 284.5 - 233.8 \propto 50.7$$ mm Hg
$$\therefore$$ x $$\propto$$ 33.8 mm Hg
Therefore, (a $$-$$ x) $$\propto$$ (233.8 $$-$$ 33.8) mm Hg
i.e., (a $$-$$ x) $$\propto$$ 200 mm Hg
$$\therefore$$ $$k = {{2.303} \over t}\log {a \over {a - x}} = {{2.303} \over {30}}\log {{233.8} \over {200}}$$
or, k = 5.21 $$\times$$ 10$$-$$3 min$$-$$1
Comments (0)


