JEE Advance - Chemistry (1990 - No. 4)
Explanation
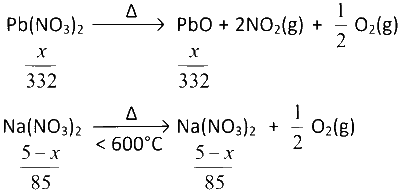
Note : 
Let, weight of $$Pb{(N{O_3})_2}$$ in the mixture = x gm
Then weight of $$NaN{O_3}$$ in the mixture $$ = (5 - x)$$ gm
$$\therefore$$ Moles of $$Pb{(N{O_3})_2} = {x \over {332}}$$
and moles of $$NaN{O_3} = {{5 - x} \over {85}}$$
$$\therefore$$ Moles of $$PbO = {x \over {332}}$$
and moles of $$NaN{O_2} = {{5 - x} \over {85}}$$
Loss in weight happens as gaseous substance removed from the mixture.
$$\therefore$$ Weight of residue $$PbO$$ and $$NaN{O_2} = 100 - 28 = 72\% $$
$$\therefore$$ Weight of residue $$ = 5 \times {{72} \over {100}} = 3.6$$ gm
$$\therefore$$ Weight of $$PbO$$ + Weight of $$NaN{O_2} = 3.6$$
$$ \Rightarrow {x \over {332}} \times 224 + {{5 - x} \over {85}} \times 69 = 3.6$$
$$ \Rightarrow 224x + (5 - x) \times 69 \times 332 = 3.6 \times 85 \times 332$$
$$ \Rightarrow x = 3.324$$ g
$$\therefore$$ Weight of $$Pb{(N{O_3})_2} = 3.324$$ gm
$$\therefore$$ Weight of $$NaN{O_3} = 5 - 3.324 = 1.676$$ gm
Comments (0)


