JEE Advance - Chemistry (1985 - No. 6)
Give reasons why the ground state outermost electronic configuration of silicon is:
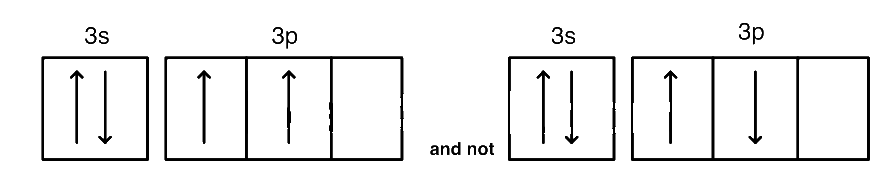

The 3p orbitals are lower in energy than 4s orbitals.
The half-filled and fully-filled configurations are more stable.
Hund's rule dictates that electrons fill orbitals individually before pairing up.
Inter-electronic repulsion is minimized when electrons occupy separate orbitals with parallel spins.
The electronic configuration maximizes exchange energy.
Comments (0)


