JEE Advance - Chemistry (1984 - No. 15)
When 16.8 g of white solid X were heated, 4.4 g of acid gas A, that turned lime water milky was driven off together with 1.8 g of a gas B which condensed to a colourless liquid.
The solid that remained, Y, dissolved in water to give an alkaline solution, which with excess barium chloride solution gave a white precipitate Z. The precipitate efferversced with acid giving off carbon dioxide. Identify A, B and Y and write down the equation for the thermal decomposition of X.
A is CO2, B is H2O, Y is Na2CO3, X is NaHCO3, Decomposition Equation: 2NaHCO3(s) → Na2CO3(s) + H2O(g) + CO2(g)
A is SO2, B is H2O, Y is Na2SO4, X is NaHSO4, Decomposition Equation: 2NaHSO4(s) → Na2SO4(s) + H2O(g) + SO3(g)
A is CO2, B is NH3, Y is Na2CO3, X is Na(NH4)CO3, Decomposition Equation: 2Na(NH4)CO3(s) → Na2CO3(s) + 2NH3(g) + H2O(g) + CO2(g)
A is CO, B is H2, Y is Na2C2, X is NaHC2, Decomposition Equation: 2NaHC2(s) → Na2C2(s) + H2(g) + 2CO(g)
A is CO2, B is H2O, Y is K2CO3, X is KHCO3, Decomposition Equation: 2KHCO3(s) → K2CO3(s) + H2O(g) + CO2(g)
Explanation
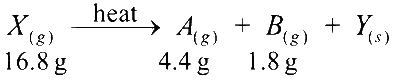
(i) Gas A turned lime water milky, which indicates the presence of carbon dioxide. Therefore, gas A is carbon dioxide (CO2).
(ii) Gas B, which condenses to a colourless liquid: This is most likely water (H2O).
(iii) $Y$ when dissolved in water yields an alkaline solution and the solution on treatment with $\mathrm{BaCl}_2$ solution forms a white ppt, of $Z$. The compound $Z$ on treatment with acid gives effervescence of $\mathrm{CO}_2$ so $Z$ and hence $Y$ must be a carbonate, $\mathrm{CO}_3^{2-}$. We can thus write $Y$ as $M \mathrm{CO}_3$ or $M_2 \mathrm{CO}_3$.
(iv) $X$ on being heated yields a carbonate $Y$, hence $\mathrm{CO}_{2(g)}$ i.e. $A$ and another gas $B$, hence it must be a bicarbonate, $\mathrm{HCO}_3^{-}$.
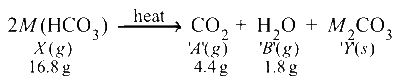
$4.4 \mathrm{~g}$ of $\mathrm{CO}_2$ is given by $M\left(\mathrm{HCO}_3\right)=16.8 \mathrm{~g}$
$44 \mathrm{~g}$ of $\mathrm{CO}_2$ is given by $M\left(\mathrm{HCO}_3\right)$
$$ =\frac{16.8}{4.4} \times 44=168 \mathrm{~g} $$
Because 2 molecules of $M\left(\mathrm{HCO}_3\right)$ are involved in the reaction so molecular weight of
$M\left(\mathrm{HCO}_3\right)=168 / 2=84$
Let the atomic weight be $M$.
Molecular weight of $M\left(\mathrm{HCO}_3\right)=M+1+12+48$$$=M+61$$
$$ \therefore M+61=84 \text { or } M=84-61=23 $$
Thus the metal must be $\mathrm{Na}$ and so the given salt $X$ is $\mathrm{Na}\left(\mathrm{HCO}_3\right)$.
Comments (0)


