JEE Advance - Chemistry (1980 - No. 10)
(b) A sample of hard water contains 20 mg of Ca++ ions per litre. How many milli-equivalent of Na2CO3 would be required to soften 1 litre of the sample?
(c) 1 gm of Mg is burnt in a closed vessel which contains 0.5gm of O2.
(i) Which reactant is left in excess?
(ii) Find the weight of the excess reactants.
(iii) How many milliliters of 0.5N H2SO4 will dissolve the residue in the vessel?
Explanation
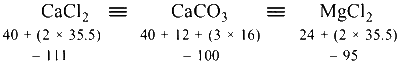
111 mg CaCl2 will give CaCO3 = 100 mg
1 mg CaCl2 will give CaCO3 = $${{100} \over {111}}$$ mg
Similarly,
1 mg MgCl2 will give $$CaC{O_3} = {{100} \over {95}}$$ mg
Total CaCO3 formed due to 1 mg CaCl2 and 1 mg of MgCl2
$$ = {{100} \over {111}} + {{100} \over {95}}$$ mg = 2 mg
CaCO3 per litre of water = 2 mg
Weight of 1 ml of water = 1 g = 103 mg
Weight of 1000 ml of water = 103 $$\times$$ 103 mg = 106 mg
2 mg of CaCO3 is present in 106 mg of water.
or 2 parts of CaCO3 in 106 part of water.
(b) $$C{a^{2 + }} + N{a_2}C{O_3} \to CaC{O_3} + 2N{a^ + }$$
Equivalent weight of $$C{a^{2 + }} = {{40} \over 2} = 20$$
1 milliequivalent of Ca2+ = 20 mg
1 milliequi. of Ca2+ = 1 millieq. of Na2CO3
$$\therefore$$ 1 milliequivalent of Na2CO3 is required to soften 1 litre of hard water.
(c) Magnesium burns in O2 to form MgO. The reaction involved is:
$$\mathop {2\,mg}\limits_{2 \times 24 = 48\,g} + \mathop {{O_2}}\limits_{32\,g} \to \mathop {2MgO}\limits_{2[24 + 16] = 80\,g} $$
(i) According to the above equation, 48 g Mg requires O2 = 32g
1 g Mg requires O2 = $${{32} \over {48}}$$ g = 0.66 g
Since the oxygen available is only 0.50 g, so whole of magnesium will not burn. Thus magnesium is in excess.
(ii) Again from the balanced equation, 32 g O2 reacts with magnesium = 48 g
0.5 g O2 reacts with magnesium
$$ = {{48} \over {32}} \times 0.5 = 0.75$$ g
Hence, weight of magnesium in excess
= (1.0 $$-$$ 0.75) = 0.25 g
(iii) Unused magnesium dissolves in H2SO4 according to the following equation:
$$\mathop {Mg}\limits_{24\,g} + \mathop {{H_2}S{O_4}}\limits_{98\,g} \to MgS{O_4} + {H_2}$$
$$\therefore$$ H2SO4 required to dissolve 0.25 g Mg
$$ = {{98} \over {24}} \times 0.25 = 1.021\,g$$
MgO formed dissolves in H2SO4 according to the following equation:
$$\mathop {MgO}\limits_{24 + 16 = 40\,g} + \mathop {{H_2}S{O_4}}\limits_{98\,g} \to MgS{O_4} + {H_2}O$$
MgO formed from 0.75
$$Mg = {{80} \over {48}} \times 0.75 = 1.25\,g$$
H2SO4 required to dissolve 1.25 g MgO
$$ = {{98} \over {40}} \times 1.25 = 3.062\,g$$
Total weight of H2SO4 required to dissolve the residue = 1.021 + 3.062 = 4.083 g
Strength of given
H2SO4 = Normality $$\times$$ Eq. wt.
= 0.5 $$\times$$ 49 = 24.5 g L$$-$$1
Hence, 24.5 g H2SO4 is present in 1000 mL 4.083 g H2SO4 is present in
$$ = {{1000} \over {24.5}} \times 4.083$$ mL = 166.65 mL
Comments (0)


