JEE Advance - Chemistry (1979 - No. 8)
Explanation
Let,
We have x gm of $$N{a_2}O$$ and y gm of $${K_2}O$$ in 0.5 gm of feldspar.
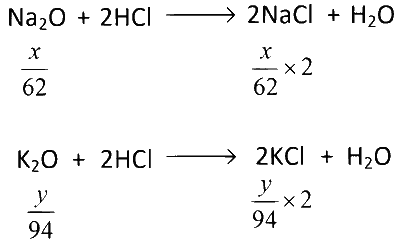
$$\therefore$$ Total NaCl obtained $$ = {x \over {62}} \times 2 \times 58.5$$ gm
and total KCl obtained $$ = {y \over {94}} \times 2 \times 74.5$$
According to the question,
$${x \over {31}} \times 58.5 + {y \over {47}} \times 74.5 = 0.118$$
$$ \Rightarrow 1.887x + 1.585y = 0.118$$ ....... (1)
Now, 2nd part of the reaction happens
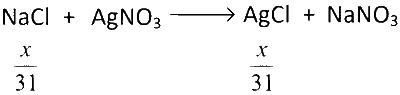
Similarly,
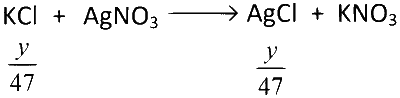
$$\therefore$$ Total moles of AgCl produced
$$ = \left( {{x \over {31}} + {y \over {47}}} \right)$$ moles
Molecular weight of AgCl = 108 + 35.5 = 143.5
Weight of $$AgCl = \left( {{x \over {31}} + {y \over {47}}} \right) \times 143.5$$
According to the question,
$$\left( {{x \over {31}} + {y \over {47}}} \right) \times 143.5 = 0.2451$$
$$ \Rightarrow 4.63x + 3.053y = 0.2451$$ ....... (2)
Solving equation (1) and (2), we get
$$x = 0.0179$$ gm
and $$y = 0.0531$$ gm
$$\therefore$$ % of $$N{a_2}O$$ in 0.5 g of feldspar $$ = {{0.0179} \over {0.5}} \times 100 = 3.58\% $$
and % of $${K_2}O$$ in 0.5 of feldspar $$ = {{0.0531} \over {0.5}} \times 100 = 10.62\% $$
Comments (0)


