JEE Advance - Chemistry (1979 - No. 7)
5 ml of a gas containing only carbon and hydrogen were mixed with an excess of oxygen (30 ml) and the mixture exploded by means of an electric spark. After the explosion, the volume of the mixed gases remaining was 25 ml. On adding a concentrated solution of potassium hydroxide, the volume further diminished to 15 ml of the residual gas being pure oxygen. All volumes have been reduced to N.T.P. Calculate the molecular formula of the hydrocarbon gas.
CH4
C2H6
C2H4
C3H8
C3H6
Explanation
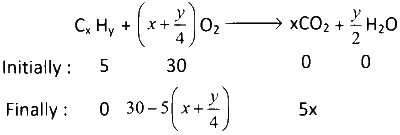
After explosion volume of mixed remain gas of $${V_{{O_2}}} + {V_{C{O_2}}} = 25$$ ml
$$\therefore$$ $$30 - 5\left( {x + {y \over 4}} \right) + 5x = 25$$
$$ \Rightarrow 30 - 5x - {{5y} \over 4} + 5x = 25$$
$$ \Rightarrow 30 - {{5y} \over 4} = 25$$
$$ \Rightarrow {{5y} \over 4} = 5$$
$$ \Rightarrow y = 4$$
Now $$KOH$$ is added to the mixture. As $$KOH$$ is basic nature so it will absorb $$C{O_2}$$. After adding $$KOH$$ volume reduce to 15 ml from 25 ml.
$$\therefore$$ Absorbed $$C{O_2}$$ by $$KOH$$ $$ = 25 - 15 = 10$$ ml
$$\therefore$$ $$5x = 10$$
$$ \Rightarrow x = 2$$
$$\therefore$$ Molecular Formula of hydrocarbon gas $$ = {C_2}{H_4}$$
Comments (0)


