JEE MAIN - Physics (2009 - No. 21)
Two moles of helium gas are taken over the cycle $$ABCD,$$ as shown in the $$P$$-$$T$$ diagram.
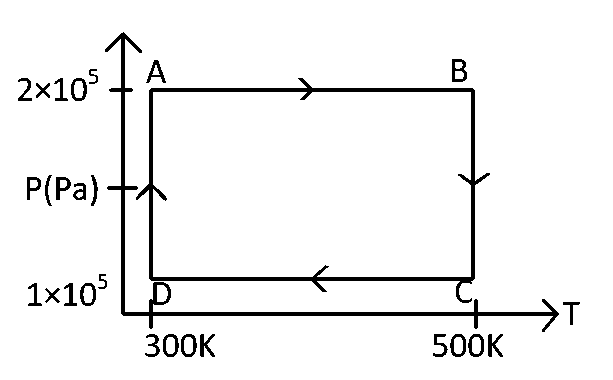

Assuming the gas to be ideal the work done on the gas in taking it from $$A$$ to $$B$$ is :
$$300$$ $$R$$
$$400$$ $$R$$
$$500$$ $$R$$
$$200$$ $$R$$
Explanation
$$A$$ to $$B$$ is an isobaric process. The work done
$$W = nR\left( {{T_2} - {T_1}} \right) = 2R\left( {500 - 300} \right) = 400R$$
$$W = nR\left( {{T_2} - {T_1}} \right) = 2R\left( {500 - 300} \right) = 400R$$
Comments (0)


