JEE MAIN - Physics (2009 - No. 19)
Two moles of helium gas are taken over the cycle $$ABCD$$, as shown in the $$P$$-$$T$$ diagram.
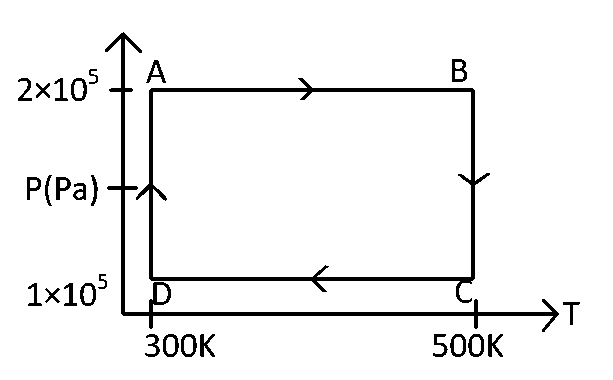

The net work done on the gas in the cycle $$ABCDA$$ is:
$$276$$ $$R$$
$$1076$$ $$R$$
$$1904$$ $$R$$
zero
Explanation
The net work in the cycle $$ABCD$$ is
$$W = {W_{AB}} + {W_{BC}} + {W_{CD}} + {W_{DA}}$$
$$ = 400R + 2.303nRT\log {{{P_B}} \over {{P_C}}} + \left( { - 400R} \right) - 414R$$
$$ = 2.303 \times 2R \times 500\log {{2 \times {{10}^5}} \over {1 \times {{10}^5}}} - 414R$$
$$ = 693.2\,R - 414\,R = 279.2\,R$$
$$W = {W_{AB}} + {W_{BC}} + {W_{CD}} + {W_{DA}}$$
$$ = 400R + 2.303nRT\log {{{P_B}} \over {{P_C}}} + \left( { - 400R} \right) - 414R$$
$$ = 2.303 \times 2R \times 500\log {{2 \times {{10}^5}} \over {1 \times {{10}^5}}} - 414R$$
$$ = 693.2\,R - 414\,R = 279.2\,R$$
Comments (0)


