JEE MAIN - Physics (2009 - No. 1)
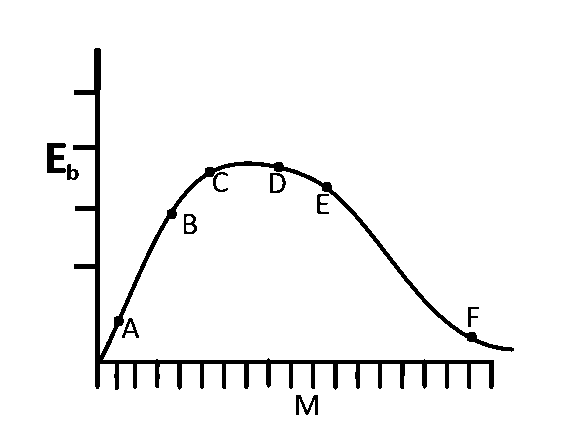
The above is a plot of binding energy per nucleon $${E_b},$$ against the nuclear mass $$M;A,B,C,D,E,F$$ correspond to different nuclei. Consider four reactions :
$$\eqalign{
& \left( i \right)\,\,\,\,\,\,\,\,\,\,A + B \to C + \varepsilon \,\,\,\,\,\,\,\,\,\,\left( {ii} \right)\,\,\,\,\,\,\,\,\,\,C \to A + B + \varepsilon \,\,\,\,\,\,\,\,\,\, \cr
& \left( {iii} \right)\,\,\,\,\,\,D + E \to F + \varepsilon \,\,\,\,\,\,\,\,\,\,\left( {iv} \right)\,\,\,\,\,\,\,\,\,F \to D + E + \varepsilon ,\,\,\,\,\,\,\,\,\,\, \cr} $$
where $$\varepsilon $$ is the energy released? In which reactions is $$\varepsilon $$ positive?
$$(i)$$ and $$(iii)$$
$$(ii)$$ and $$(iv)$$
$$(ii)$$ and $$(iii)$$
$$(i)$$ and $$(iv)$$
Explanation
For $$A + B \to C + \varepsilon ,\,\,\varepsilon $$ is positive. This is because $${E_b}$$
for $$C$$ is greater than the $${E_b}$$ for $$A$$ and $$B$$.
Again for $$F \to D + E + \varepsilon ,\varepsilon $$ is positive. This is
because $${E_b}$$ for $$D$$ and $$E$$ is greater than $${E_b}$$ for $$F.$$
for $$C$$ is greater than the $${E_b}$$ for $$A$$ and $$B$$.
Again for $$F \to D + E + \varepsilon ,\varepsilon $$ is positive. This is
because $${E_b}$$ for $$D$$ and $$E$$ is greater than $${E_b}$$ for $$F.$$
Comments (0)


