JEE MAIN - Physics (2007 - No. 29)
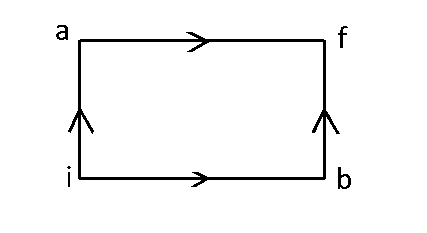
When a system is taken from state $$i$$ to state $$f$$ along the path iaf, it is found that $$Q=50$$ cal and $$W=20$$ $$cal$$. Along the path $$ibf$$ $$Q=36$$ $$cal.$$ $$W$$ along the path $$ibf$$ is

$$14$$ $$cal$$
$$6$$ $$cal$$
$$16$$ $$cal$$
$$66$$ $$cal$$
Explanation
For path iaf, $$\Delta U = Q - W = 50 - 20 = 30\,cal.$$
For path ibf, $$W = Q - \Delta U = 36 - 30 = 6\,cal.$$
For path ibf, $$W = Q - \Delta U = 36 - 30 = 6\,cal.$$
Comments (0)


