JEE MAIN - Physics (2005 - No. 39)
The temperature-entropy diagram of a reversible engine cycle is given in the figure. Its efficiency is
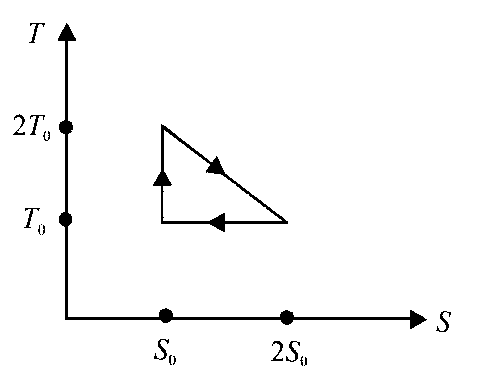

$${1 \over 4}$$
$${1 \over 2}$$
$${2 \over 3}$$
$${1 \over 3}$$
Explanation
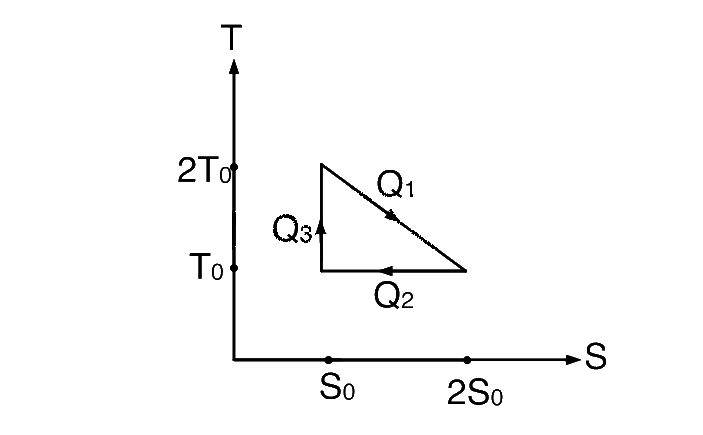
$${Q_1} = {T_0}{S_0} + {1 \over 2}{T_0}{S_0} = {3 \over 2}{T_0}{S_0}$$
$${Q_2} = {T_0}\left( {2{S_0} - {S_0}} \right)$$ $$ = {T_0}{S_0}$$
and $${Q_3} = 0$$
$$\eta = 1 - {{{Q_2}} \over {{Q_1}}} = 1 - {{{T_0}{S_0}} \over {{3 \over 2}{T_0}{S_0}}} = {1 \over 3}$$
Comments (0)


