JEE MAIN - Physics (2005 - No. 37)
A system goes from $$A$$ to $$B$$ via two processes $$I$$ and $$II$$ as shown in figure. If $$\Delta {U_1}$$ and $$\Delta {U_2}$$ are the changes in internal energies in the processes $$I$$ and $$II$$ respectively, then
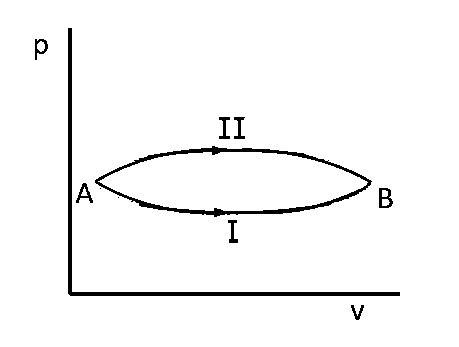

relation between $$\Delta {U_1}$$ and $$\Delta {U_2}$$ can not be determined
$$\Delta {U_1} = \Delta {U_2}$$
$$\Delta {U_2} < \Delta {U_1}$$
$$\Delta {U_2} > \Delta {U_1}$$
Explanation
Change in internal energy do not depend upon the path followed by the process. It only depends on initial and final states $$i.e.,$$ $$\Delta U{}_1 = \Delta {U_2}$$
Comments (0)


