JEE MAIN - Physics (2005 - No. 3)
The diagram shows the energy levels for an electron in a certain atom. Which transition shown represents the emission of a photon with the most energy?
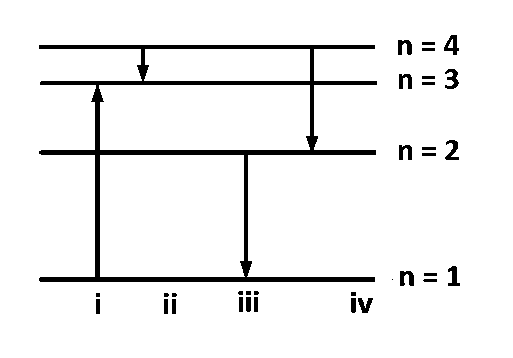

$$iv$$
$$iii$$
$$ii$$
$$i$$
Explanation
KEY CONCEPT : $$E = Rhc\left[ {{1 \over {{n_1}^2}} - {1 \over {{n_2}^2}}} \right]$$
$$E$$ will be maximum for the transition for which
$$\left[ {{1 \over {{n_1}^2}} - {1 \over {{n_2}^2}}} \right]$$ is maximum. Here $${n_2}$$ is the higher energy level
Clearly, $$\left[ {{1 \over {{n_1}^2}} - {1 \over {{n_2}^2}}} \right]$$ is maximum for the third
transition, i.e. $$2 \to 1.$$ $$I$$ transition represents the absorption of energy.
$$E$$ will be maximum for the transition for which
$$\left[ {{1 \over {{n_1}^2}} - {1 \over {{n_2}^2}}} \right]$$ is maximum. Here $${n_2}$$ is the higher energy level
Clearly, $$\left[ {{1 \over {{n_1}^2}} - {1 \over {{n_2}^2}}} \right]$$ is maximum for the third
transition, i.e. $$2 \to 1.$$ $$I$$ transition represents the absorption of energy.
Comments (0)


