JEE MAIN - Chemistry (2012 - No. 20)
Ortho–Nitrophenol is less soluble in water than p– and m– Nitrophenols because :
o–Nitrophenol is more volatile in steam than those of m – and p–isomers
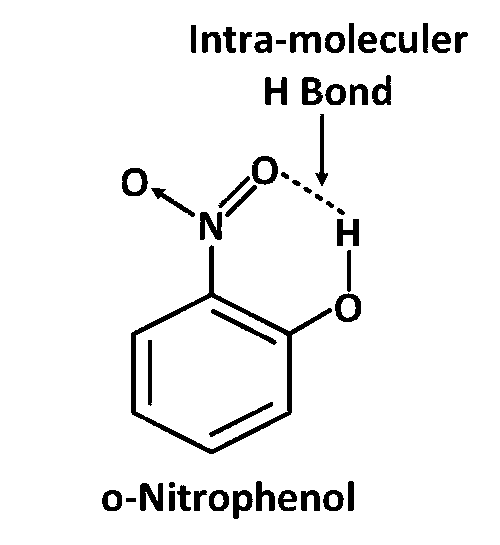
o–Nitrophenol shows Intramolecular H–bonding
o–Nitrophenol shows Intermolecular H–bonding
Melting point of o–Nitrophenol is lower than those of m–and p–isomers.
Explanation
As in ortho-Nitrophenol H of -OH is already in Intra-moleculer H Bond and become stable so it will not perticipate in H bonding with H2O by breaking this H bonding. That is why solublity of Ortho–Nitrophenol is less in water.
para– and meta– Nitrophenols both shows inter-moleculer H bonding with H2O. That is why both are more soluble in water.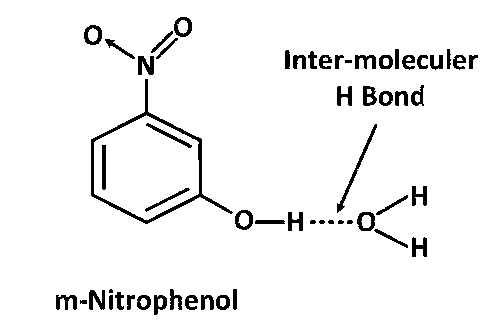
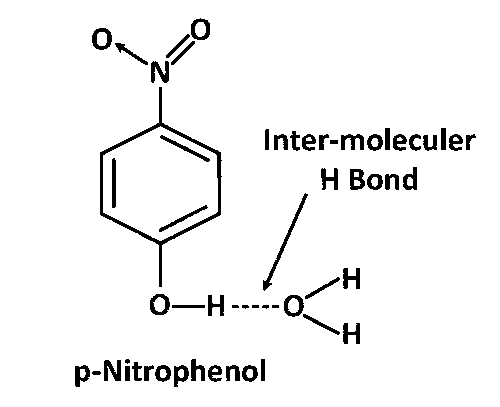

para– and meta– Nitrophenols both shows inter-moleculer H bonding with H2O. That is why both are more soluble in water.


Comments (0)


