JEE MAIN - Chemistry (2012 - No. 10)
In the given transformation, which of the following is the most appropriate reagent ?
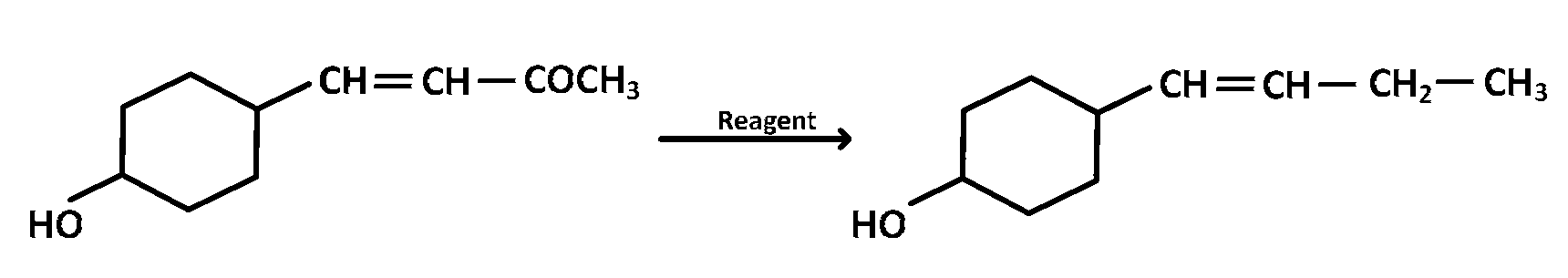

$$N{H_2}N{H_2},\mathop {OH}\limits^\Theta $$
$$Zn - Hg/HCl$$
$$Na,Liq\,N{H_3}$$
$$NaB{H_4}$$
Explanation
Aldehydes and ketones can be reduced to hydrocarbons by the action $$(i)$$ of amalgamated zinc and concentrated hydrochloric acid (Clemmensen reduction), or $$(b)$$ of hydrazine $$\left( {N{H_2}N{H_2}} \right)$$ and a strong base like $$NaOH,KOH$$ or potassium tert-butoxide in a high-boiling alcohol like ethylene glycol or triethylene glycol (Wolf-Kishner reduction )
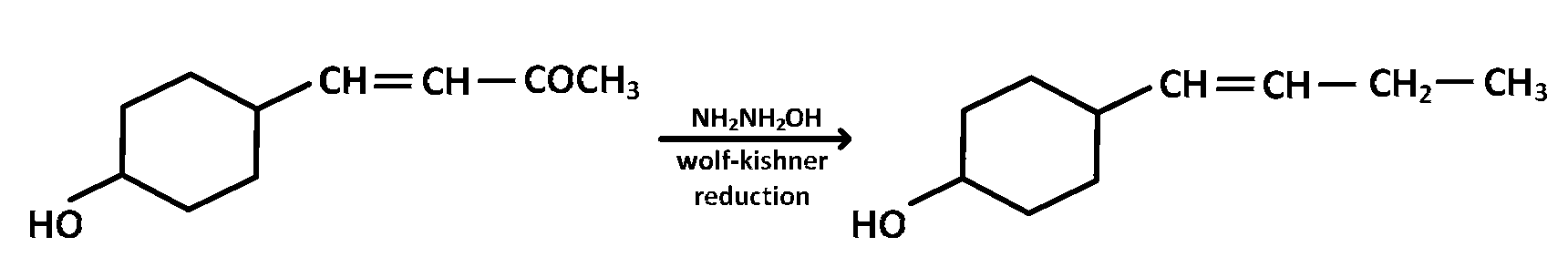
$$-OH$$ group and alkene are acid - sensitive groups so clemmensen reduction can not be used. Acid sensitive substrate should be reacted in the Wolf-Kishner reduction which utilise strongly basic conditions.

$$-OH$$ group and alkene are acid - sensitive groups so clemmensen reduction can not be used. Acid sensitive substrate should be reacted in the Wolf-Kishner reduction which utilise strongly basic conditions.
Comments (0)


