JEE MAIN - Chemistry (2011 - No. 17)
The magnetic moment (spin only) of [NiCl4]2− is
5.46 BM
2.82 BM
1.41 BM
1.82 BM
Explanation
$${\left[ {NiC{l_4}} \right]^{2 - }}\,\left( {{d^8}} \right)$$
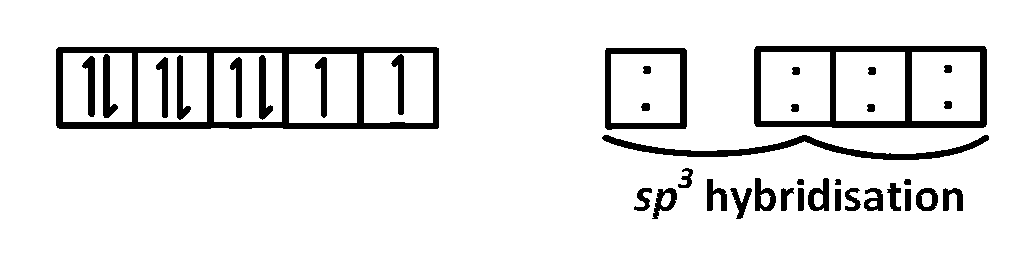
i.e. the number of unpaired electrons in $${\left[ {NiCl{}_4} \right]^{2 - }}\,\,$$ is $$2.$$
$$n = \sqrt {n\left( {n + 2} \right)} = \sqrt {2\left( 4 \right)} $$
$$ = 2\sqrt 2 = 2 \times 1.41$$
$$ = 2.82\,BM$$

i.e. the number of unpaired electrons in $${\left[ {NiCl{}_4} \right]^{2 - }}\,\,$$ is $$2.$$
$$n = \sqrt {n\left( {n + 2} \right)} = \sqrt {2\left( 4 \right)} $$
$$ = 2\sqrt 2 = 2 \times 1.41$$
$$ = 2.82\,BM$$
Comments (0)


