JEE MAIN - Chemistry (2010 - No. 16)
Which one of the following has an optical isomer ?
(en = ethylenediamine)
(en = ethylenediamine)
[Zn (en) (NH3)2]2+
[Co (en)3]3+
[Co (H2O)4 en]3+
[Zn (en)2]2+
Explanation
For a substance to be optical isomers following conditions should be fulfilled
$$(a)\,\,\,$$ A coordination compound which can rotate the plane of polarized light is said to be optically active.
$$(b)\,\,\,$$ When the coordination compounds have same formula but differ in their abilities to rotate directions of the plane of polarized light are said to exhibit optical isomerism and the molecules are optical isomers. The optical isomers are pair of molecules which are non-super-imposable mirror images of each other.
$$(c)\,\,\,$$ This is due to the absence of elements of symmetry in the complex.
$$(d)\,\,\,$$ Optical isomerism is expected in tetrahedral complexes of the type $$Mabcd.$$
Based on this only option $$(2)$$ shows optical isomerism $${\left[ {Co{{\left( {en} \right)}_3}} \right]^{3 + }}$$
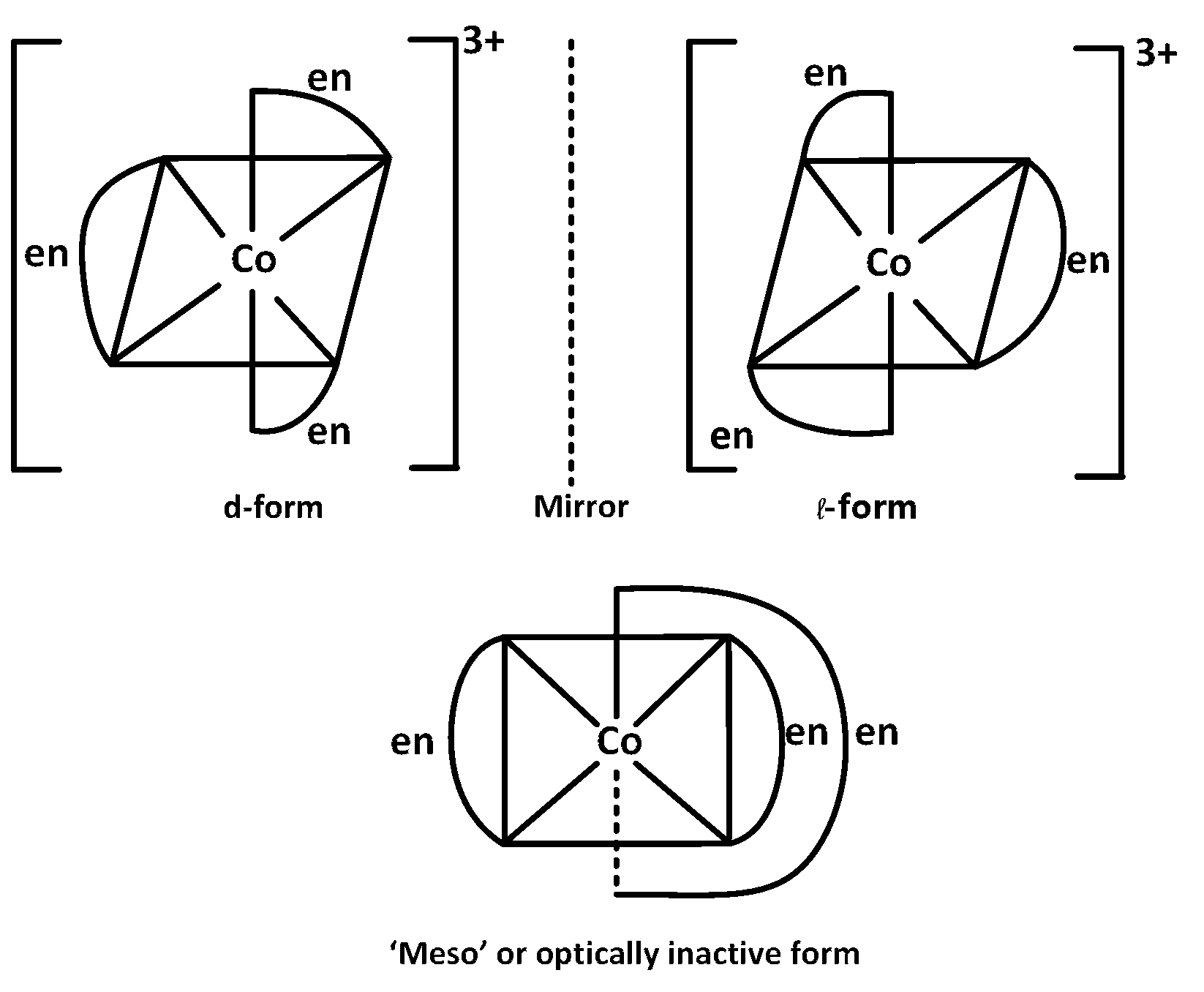
Complexes of $$Z{n^{ + + }}\,\,$$ cannot show optical isomerism as they are tetrahedral complexes with plane of symmetry.
$${\left[ {Co{{\left( {{H_2}O} \right)}_4}\left( {en} \right)} \right]^{3 + }}\,\,$$ have two planes of symmetry hence it is also optically inactive.
Hence the formula of the complex is $$\left[ {Co{{\left( {N{H_3}} \right)}_6}} \right]C{l_3}$$
$$(a)\,\,\,$$ A coordination compound which can rotate the plane of polarized light is said to be optically active.
$$(b)\,\,\,$$ When the coordination compounds have same formula but differ in their abilities to rotate directions of the plane of polarized light are said to exhibit optical isomerism and the molecules are optical isomers. The optical isomers are pair of molecules which are non-super-imposable mirror images of each other.
$$(c)\,\,\,$$ This is due to the absence of elements of symmetry in the complex.
$$(d)\,\,\,$$ Optical isomerism is expected in tetrahedral complexes of the type $$Mabcd.$$
Based on this only option $$(2)$$ shows optical isomerism $${\left[ {Co{{\left( {en} \right)}_3}} \right]^{3 + }}$$

Complexes of $$Z{n^{ + + }}\,\,$$ cannot show optical isomerism as they are tetrahedral complexes with plane of symmetry.
$${\left[ {Co{{\left( {{H_2}O} \right)}_4}\left( {en} \right)} \right]^{3 + }}\,\,$$ have two planes of symmetry hence it is also optically inactive.
Hence the formula of the complex is $$\left[ {Co{{\left( {N{H_3}} \right)}_6}} \right]C{l_3}$$
Comments (0)


