JEE MAIN - Chemistry (2010 - No. 14)
From amongst the following alcohols the one that would react fastest with conc. HCl and anhydrous
ZnCl2, is
2–Butanol
2–Methylpropan–2–ol
2–Methylpropanol
1–Butanol
Explanation
Tertiary alcohols react fastest with conc. $$HCl$$ and anhydrous $$ZnC{l_2}$$ (lucas reagent) as its mechanism proceeds through the formation of stable tertiary carbocation.
Mechanism
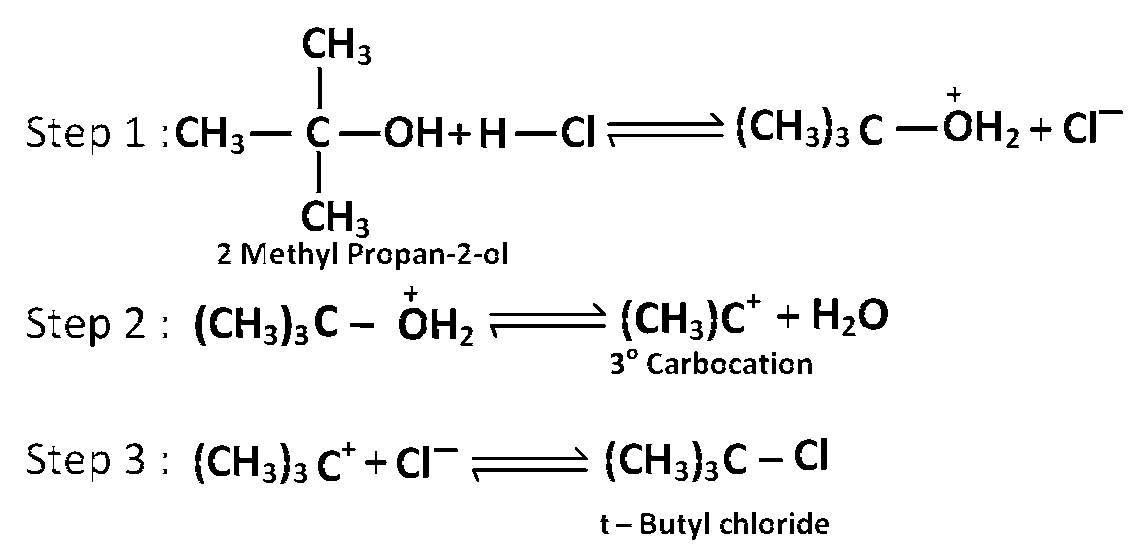
Mechanism

Comments (0)


