JEE MAIN - Chemistry (2010 - No. 13)
Consider the following bromides :
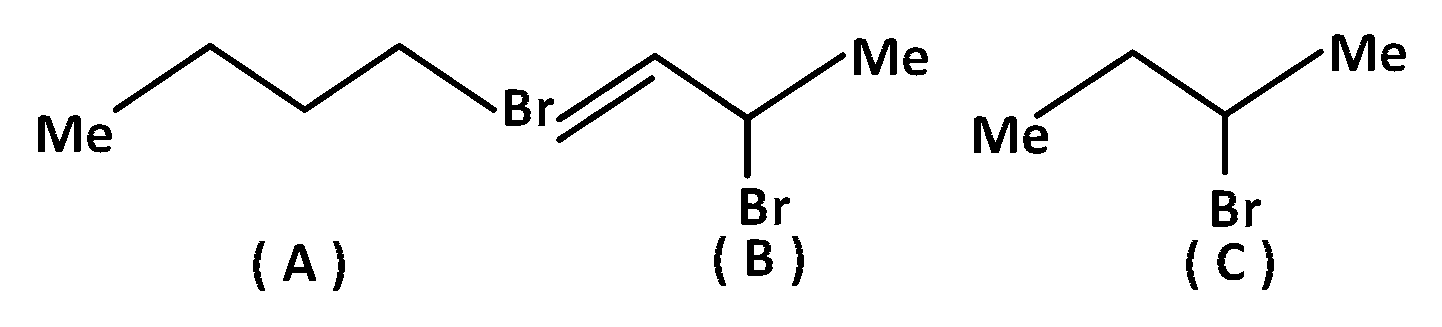
The correct order of $${S_N}1$$ reactive is

The correct order of $${S_N}1$$ reactive is
$$B > C > A$$
$$B > A > C$$
$$C > B > A$$
$$A > B > C$$
Explanation
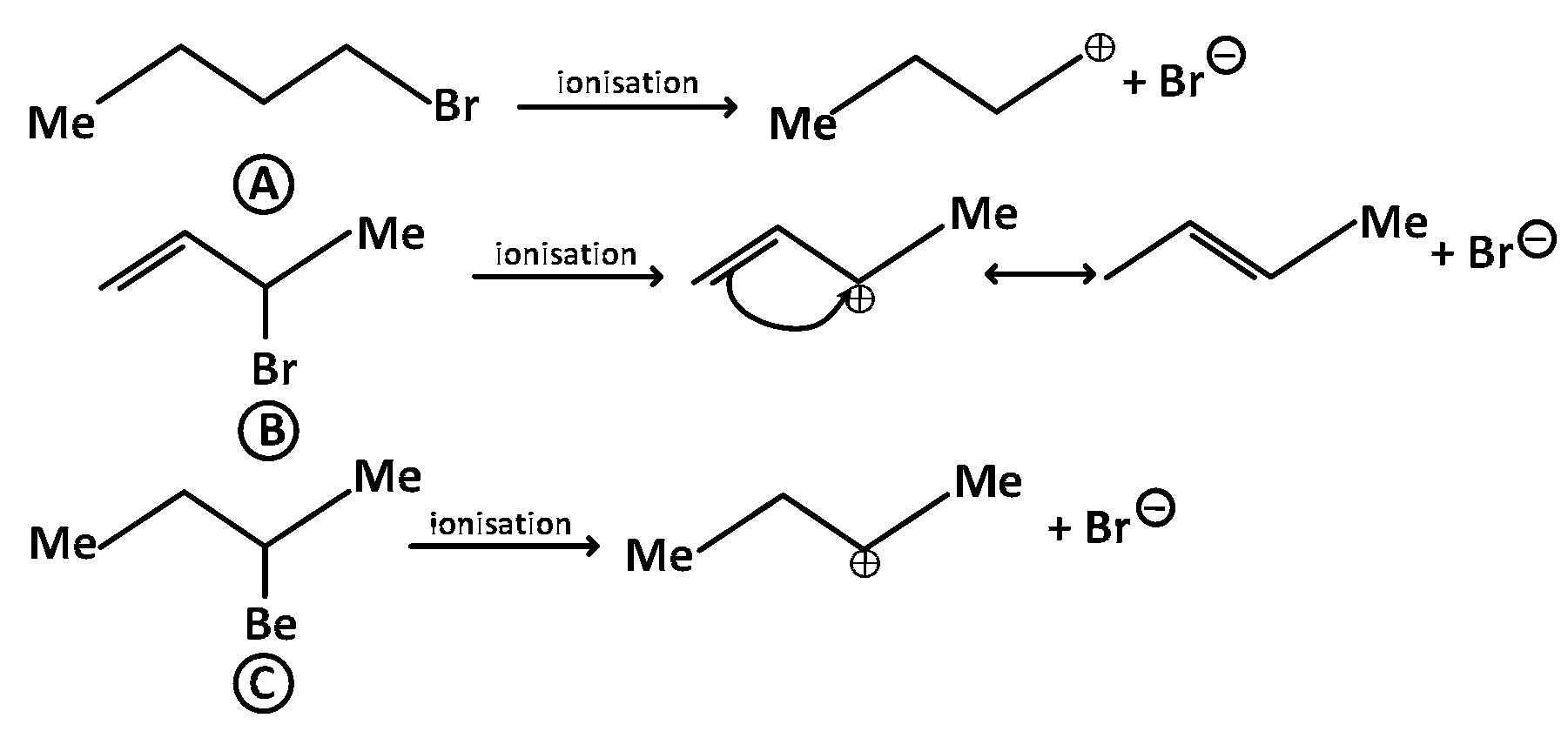
Since $${S_N}1$$ reactions involve the formation of carbocation as intermediate in the rate determining step, more is the stability of carbocation higher will be reactivity of alkyl halides towards $${S_N}1$$ route. Now we know that stability of carbocations follows the order : $${3^ \circ } > {2^ \circ } > {1^ \circ },\,\,\,$$ $${S_N}1$$ reactivity should also follow the same order. $${3^ \circ } > {2^ \circ } > 1 > $$ Methyl ($${{S_N}1}$$ reactivity)
Comments (0)


