JEE MAIN - Chemistry (2009 - No. 17)
In context with the transition elements, which of the following statements is incorrect ?
In addition to the normal oxidation states, the zero oxidation state is also shown by these elements in complexes.
In the highest oxidation states, the transition metal show basic character and form cationic complexes.
In the highest oxidation states of the first five transition elements (Sc to Mn), all the 4s and 3d electrons are used for bonding
Once the d5 configuration is exceeded, the tendency to involve all the 3d electrons in bonding
decreases.
Explanation
Lower oxidation state of an element forms more basic oxide and hydroxide, while the higher oxidation state will form more acidic oxide/hydroxide. For example,
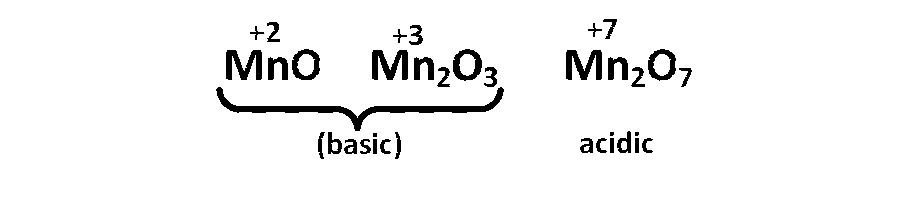
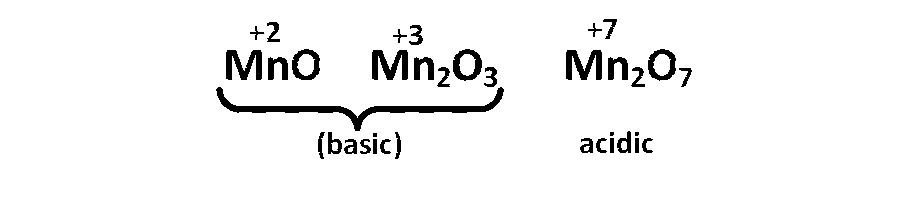
Comments (0)


