JEE MAIN - Chemistry (2008 - No. 3)
The hydrocarbon which can react with sodium in liquid ammonia is
CH3CH2CH2C≡CCH2CH2CH3
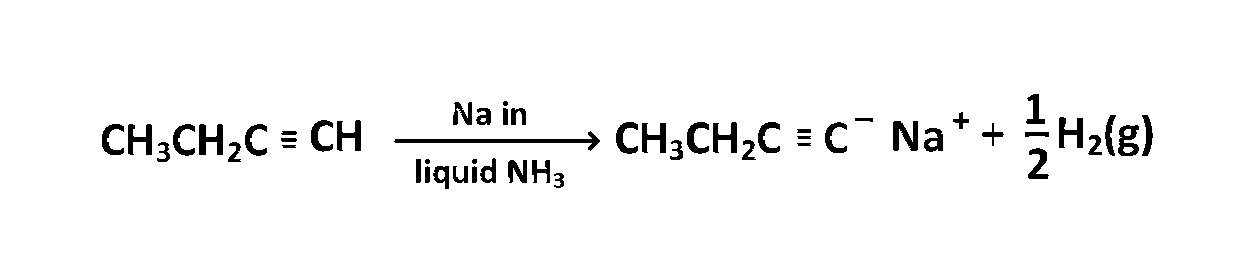
CH3CH2C≡CH
CH3CH=CHCH3
CH3CH2C≡CCH2CH3
Explanation
Alkynes having terminal $$ - C \equiv H$$ react with Na in liquid ammonia to yield $${H_2}$$ gas of the given compounds $$C{H_3}C{H_2}C \equiv CH$$ can react with Na in liquid $$N{H_3}$$ so the correct answer is $$(b).$$

Comments (0)


