JEE MAIN - Chemistry (2008 - No. 16)
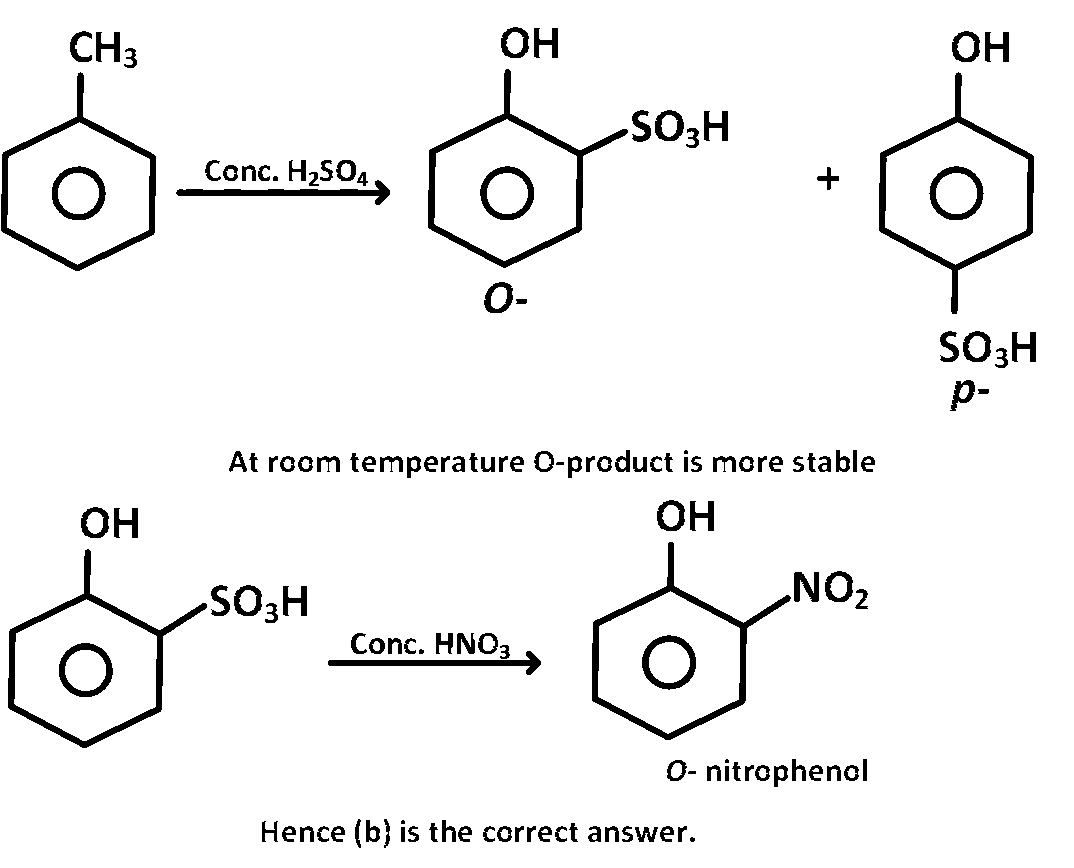
Phenol, when it first reacts with concentrated sulphuric acid and then with concentrated nitric acid,
gives
2,4,6-trinitrobenzene
o-nitrophenol
p-nitrophenol
nitrobenzene
Explanation
Phenol on reaction with conc. $${H_2}S{O_4}$$ gives a mixture of $$o$$- and $$p$$- products (i.e., $$ - S{O_3}H$$ group, occupies $$o$$-, $$p$$- position). At room temperature $$o$$-product is more stable, which on treatment with conc. $$HN{O_3}$$ will yield $$o$$-nitrophenol.

Comments (0)


