JEE MAIN - Chemistry (2008 - No. 14)
The electrophile, $${E^ \oplus }$$ attacks the benzene ring to generate the intermediate $$\sigma - $$complex. Of the following, which $$\sigma - $$complex is lowest energy?
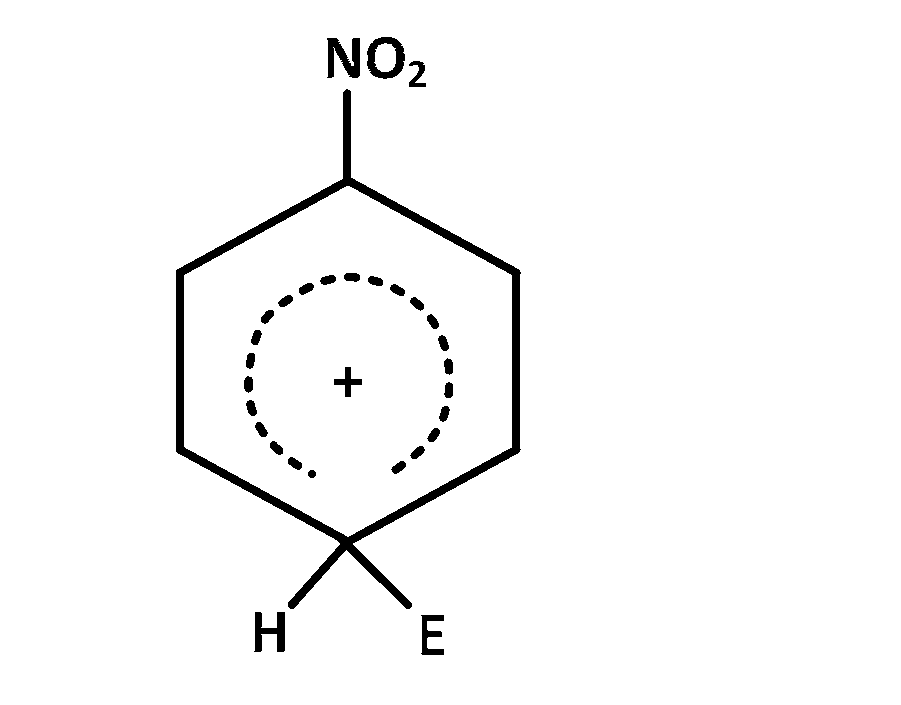
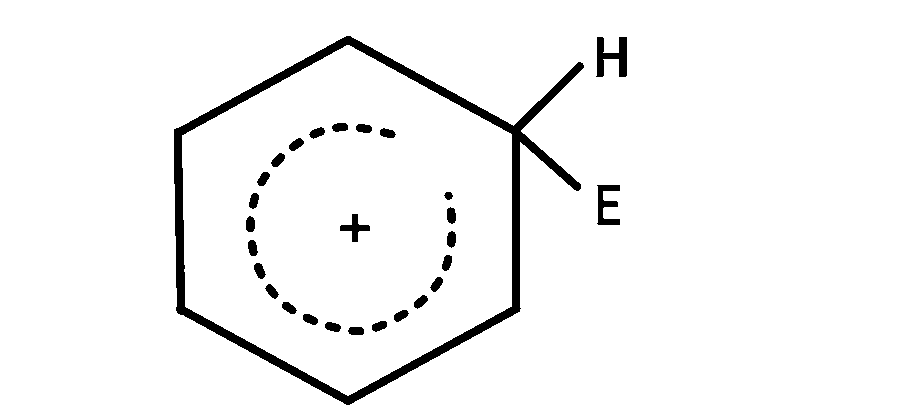
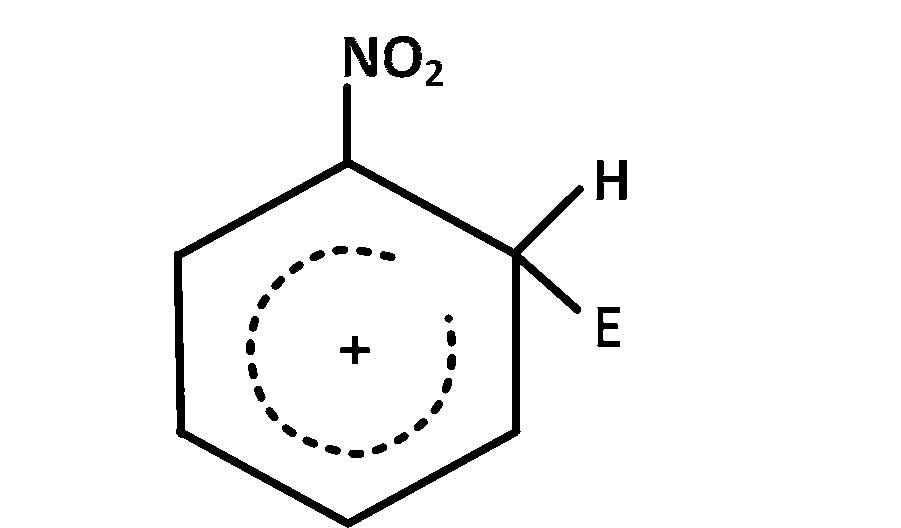
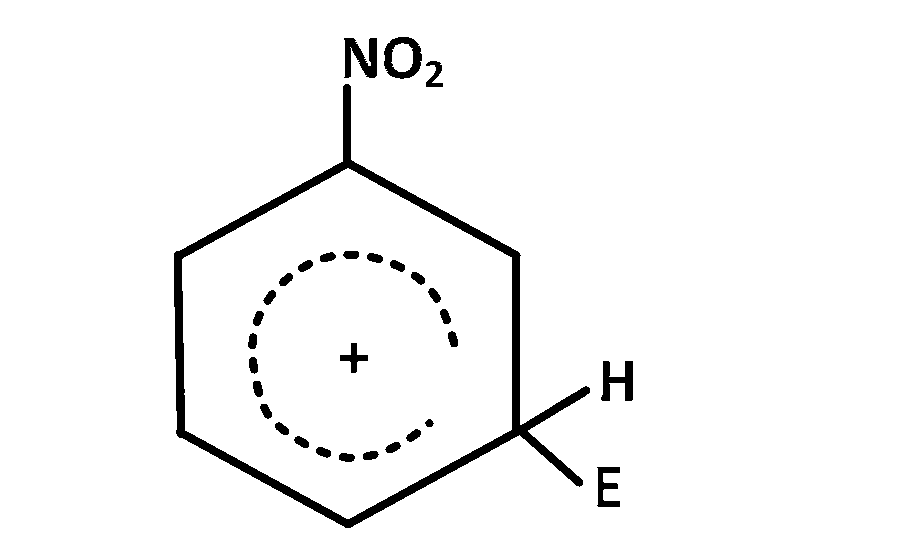
Explanation
In option $$(b)$$ the complex formed is with benzene where as in other cases it is formed with nitrobenzene with $$ - N{O_2}$$ group in different position $$\left( {O - ,m - ,p - } \right).$$ The complex formed with nitrobenzene in any position of $$ - N{O_2}$$ group is less stable than the complex formed with benzene so the correct answer is $$(b)$$
NOTE : The most stable complex has lowest energy.
NOTE : The most stable complex has lowest energy.
Comments (0)


