JEE MAIN - Chemistry (2006 - No. 50)
In which of the following molecules/ions are all the bonds not equal?
XeF4
$$BF_4^−$$
SF4
SiF4
Explanation
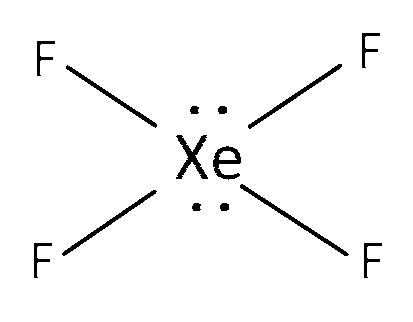
(a) XeF4 is sp3d2 hybridised with 4 bond pairs and 1 lone pair and structure is square planar. Here all the bond lengths are equal.
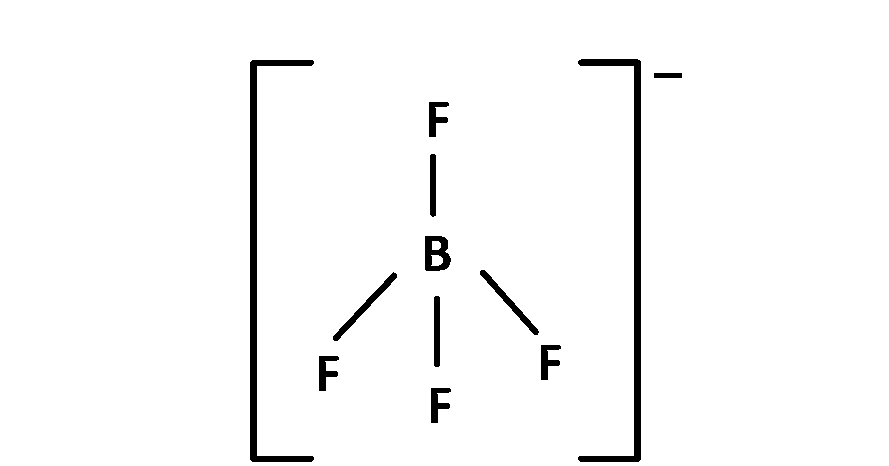
(b) $$\,\,\,\,$$ BF$$_4^ - $$, 4 bond pair present so angle is 109o 28' and sp3 hybridised. So structure is regular tetrahedral. Here all the bond lengths are equal.
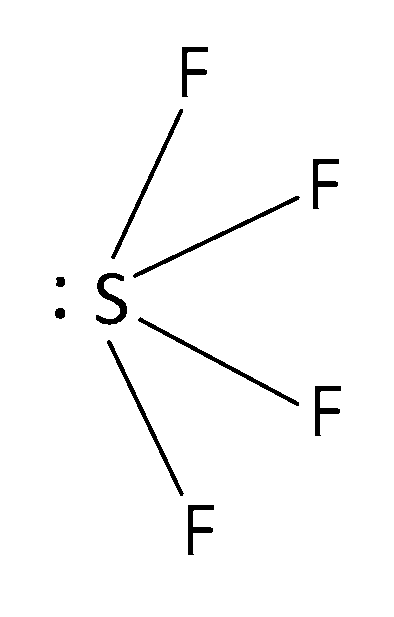
(c) SF4 is sp3d hybridised with 4 bond pairs and 1 lone pair and its expected trigonal bipyramidal geometry gets distorted due to presence of a lone pair of electrons and it becomes distorted tetrahedral or see-saw with the bond angles equal to < 120o and 179o instead of the expected angles of 120o and 180o respectively. Here axial and equitorial both bonds are presents. And we know axial bonds are longer and weaker.
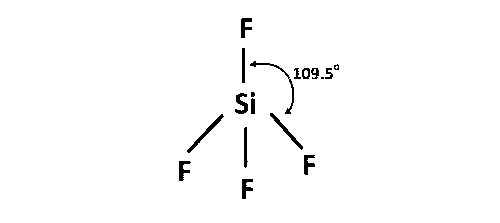
(d) SiF4 is sp3 hybridisation and regular tetrahedral geometry. Here all the bond lengths are equal.

(b) $$\,\,\,\,$$ BF$$_4^ - $$, 4 bond pair present so angle is 109o 28' and sp3 hybridised. So structure is regular tetrahedral. Here all the bond lengths are equal.

(c) SF4 is sp3d hybridised with 4 bond pairs and 1 lone pair and its expected trigonal bipyramidal geometry gets distorted due to presence of a lone pair of electrons and it becomes distorted tetrahedral or see-saw with the bond angles equal to < 120o and 179o instead of the expected angles of 120o and 180o respectively. Here axial and equitorial both bonds are presents. And we know axial bonds are longer and weaker.

(d) SiF4 is sp3 hybridisation and regular tetrahedral geometry. Here all the bond lengths are equal.

Comments (0)


