JEE MAIN - Chemistry (2006 - No. 41)
Nickel (Z = 28) combines with a uninegative monodentate ligand X– to form a paramagnetic complex [NiX4]2− . The number of unpaired electron(s) in the nickel and geometry of this complex ion are,
respectively
one, tetrahedral
two, tetrahedral
one, square planar
two, square planar
Explanation
$${\left[ {Ni{X_4}} \right]^{2 - }},$$ the electronic configuration of $$N{i^{2 + }}$$ is
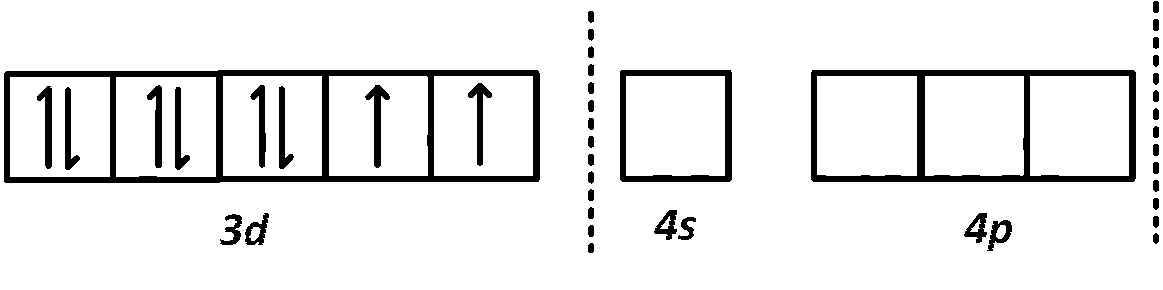
It contains two unpaired electrons and the hybridisation is $$s{p^3}$$ (tetrahedral).

It contains two unpaired electrons and the hybridisation is $$s{p^3}$$ (tetrahedral).
Comments (0)


