JEE MAIN - Chemistry (2006 - No. 29)
Among the following mixtures, dipole-dipole as the major interaction, is present in
benzene and ethanol
acetonitrile and acetone
KCl and water
benzene and carbon tetrachloride
Explanation
Acetonitrile $$\mathop {\left( {C{H_3}} \right.}\limits^{\delta + } \,$$ $$\,\, - \,\,\,C \equiv \,\,$$ $$\mathop {\left. N \right)}\limits^{\delta - } $$ and acetone
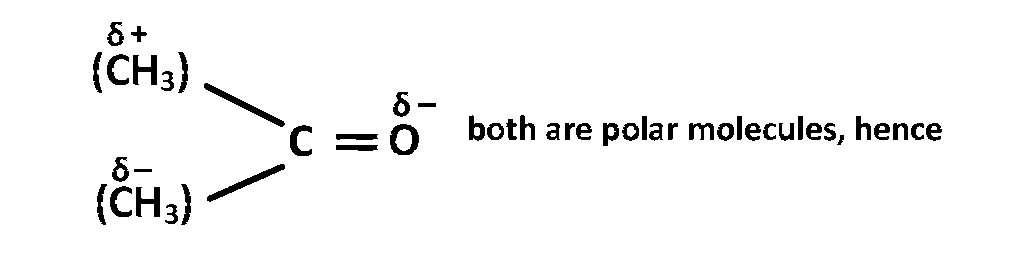
dipole-dipole interaction exist between them. Between $$KCl$$ and water ion-dipole interaction is found and in Benzene - ethanol, Benzene is non polar but ethanol is polar so it create dipole - induced dipole interaction
Benzene$$-$$Carbon tetra chloride, both are non polar so one create instantaneous dipole and other gets induced by it.
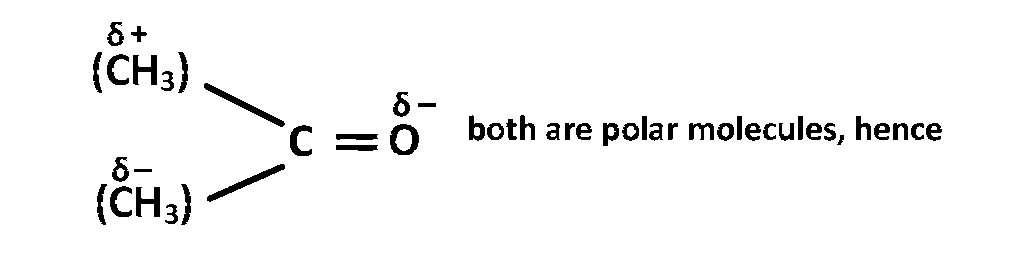
dipole-dipole interaction exist between them. Between $$KCl$$ and water ion-dipole interaction is found and in Benzene - ethanol, Benzene is non polar but ethanol is polar so it create dipole - induced dipole interaction
Benzene$$-$$Carbon tetra chloride, both are non polar so one create instantaneous dipole and other gets induced by it.
Comments (0)


