JEE MAIN - Chemistry (2006 - No. 26)
The correct order of increasing acid strength of the compounds
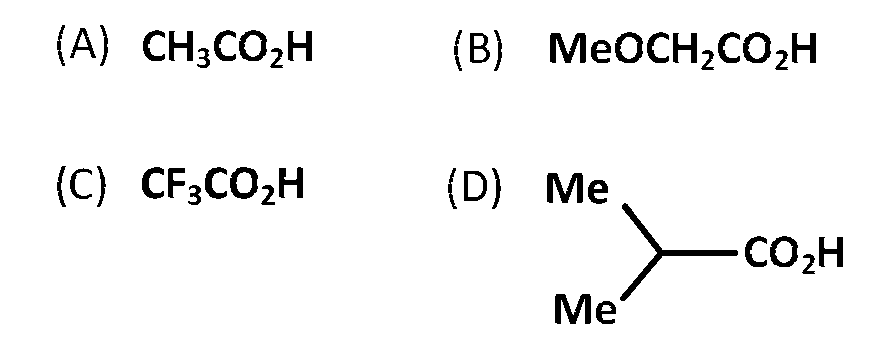
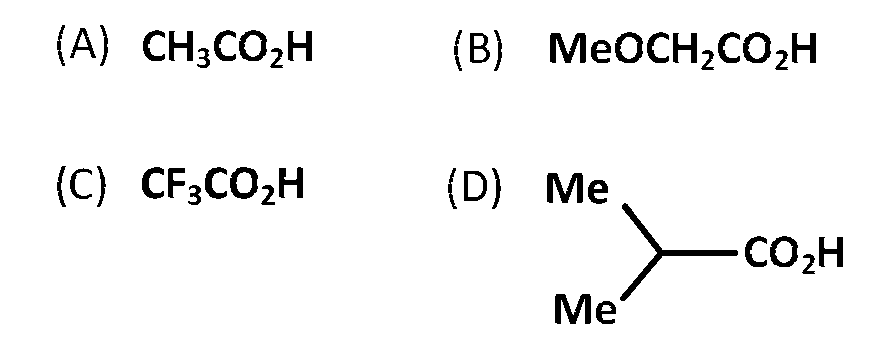
$$D < A < B < C$$
$$A < D < B < C$$
$$B < D < A < C$$
$$D < A < C < B$$
Explanation
The correct order of increasing acid strength
$$C{F_3}.COOH > MeOC{H_2}COOH > C{H_3}COOH > {(Me)_2}CH.COOH$$
[NOTE : Electron withdrawing groups increase the acid strength and electron donating groups decrease the acid strength.]
$$C{F_3}.COOH > MeOC{H_2}COOH > C{H_3}COOH > {(Me)_2}CH.COOH$$
[NOTE : Electron withdrawing groups increase the acid strength and electron donating groups decrease the acid strength.]
Comments (0)


