JEE MAIN - Chemistry (2005 - No. 41)
The lanthanide contraction is responsible for the fact that :
Zr and Y have about the same radius
Zr and Nb have similar oxidation
state
Zr and Hf have about the same radius
Zr and Zn have the same oxidation
Explanation
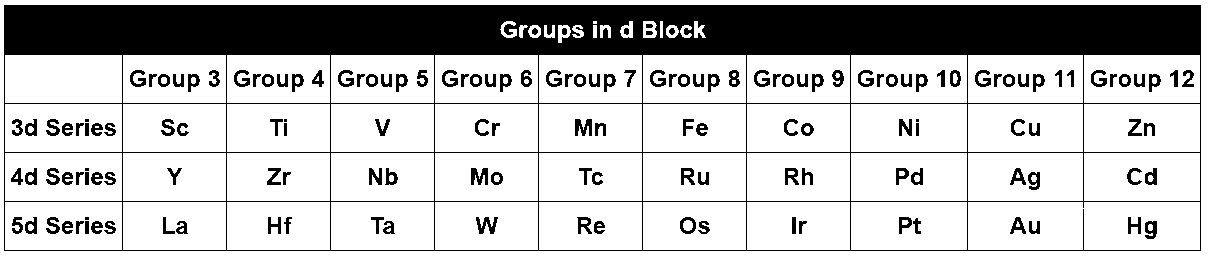
In the entire d-block,
Only in group 3, the size increase from top to bottom due to increase of no of shells in an atom. That is why size of Sc < Y < La. And shell size in group 3 is 3d < 4d < 5d.
But from group 4 to 12, the size of 3d < 4d but 4d $$ \cong $$ 5d due to lanthanide contraction. That is why Zr and Hf have about the same radius.
Comments (0)


