JEE MAIN - Chemistry (2005 - No. 27)
A schematic plot of $$ln$$ $${K_{eq}}$$ versus inverse of temperature for a reaction is shown below
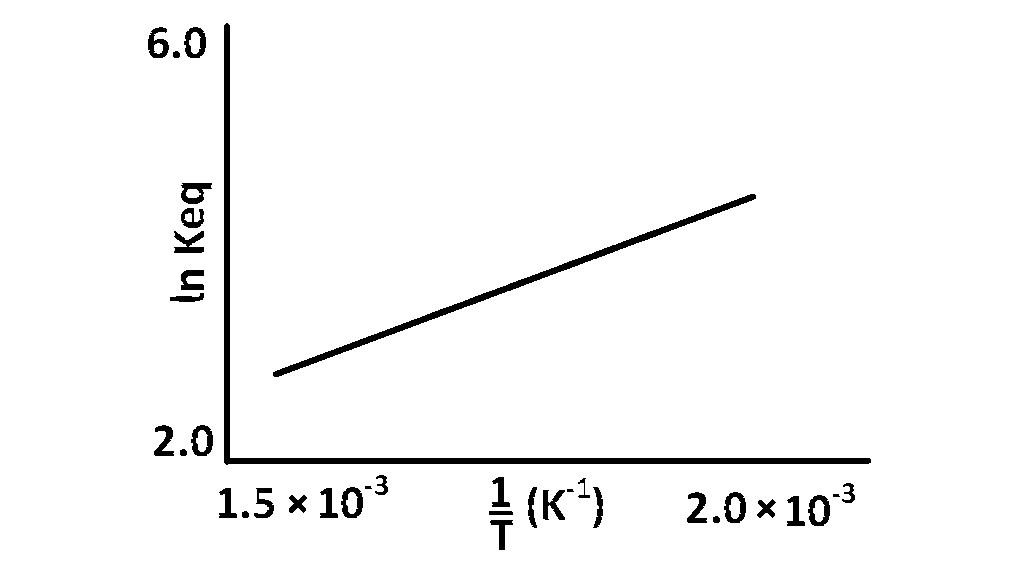
The reaction must be
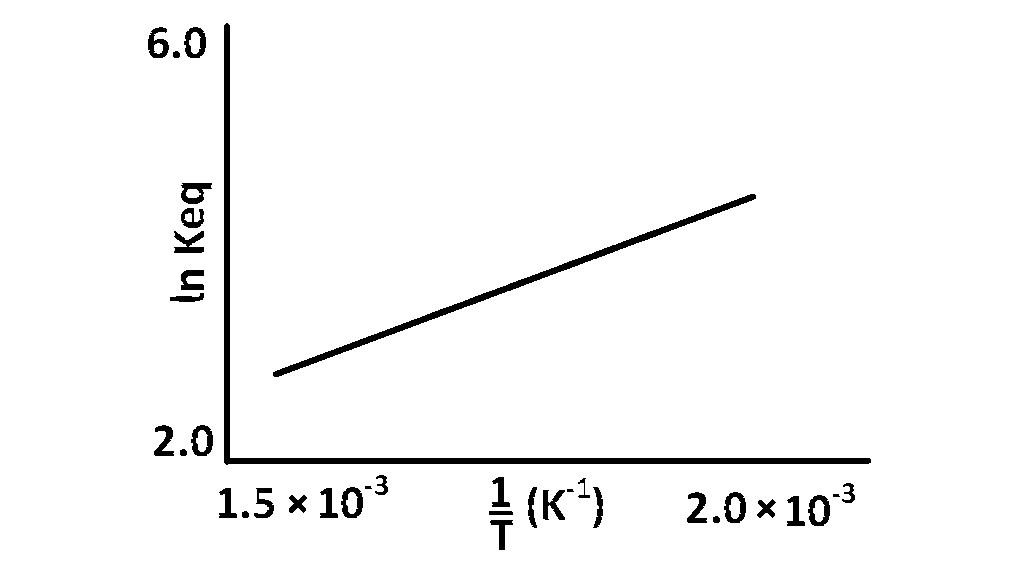
The reaction must be
highly spontaneous at ordinary temperature
one with negligible enthalpy change
endothermic
exothermic
Explanation
The graph show that reaction is exothermic.
$$\log \,k = {{ - \Delta H} \over {RT}} + 1$$
For exothermic reaction $$\Delta H < 0$$
$$\therefore$$ $$\,\,\,\,\,log\,\,k\,\,Vs{1 \over T}\,\,$$ would be negative straight line with positive slope.
$$\log \,k = {{ - \Delta H} \over {RT}} + 1$$
For exothermic reaction $$\Delta H < 0$$
$$\therefore$$ $$\,\,\,\,\,log\,\,k\,\,Vs{1 \over T}\,\,$$ would be negative straight line with positive slope.
Comments (0)


