JEE MAIN - Chemistry (2005 - No. 26)
The reaction
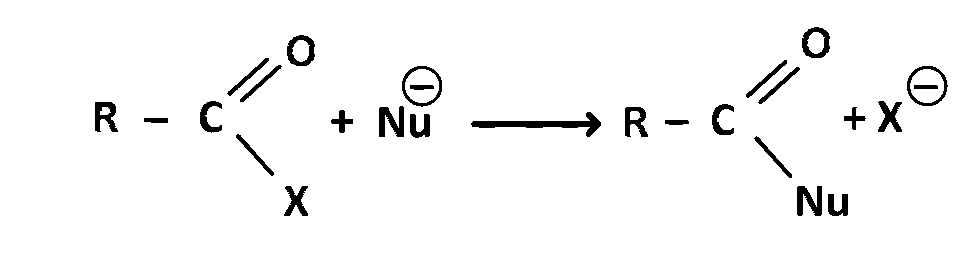
is fastest when $$X$$ is

is fastest when $$X$$ is
$$OCOR$$
$$O{C_2}{H_5}$$
$$N{H_2}$$
$$Cl$$
Explanation
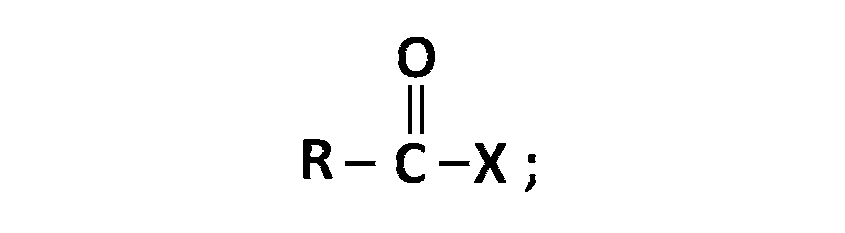
when $$X$$ is $$Cl$$ the $$C$$-$$X$$ bond is more polar and ionic which leaves the compound more reactive for nucleophilic substitution reaction.
Comments (0)


