JEE MAIN - Chemistry (2005 - No. 25)
The decreasing order of nucleophilicity among the nucleophiles
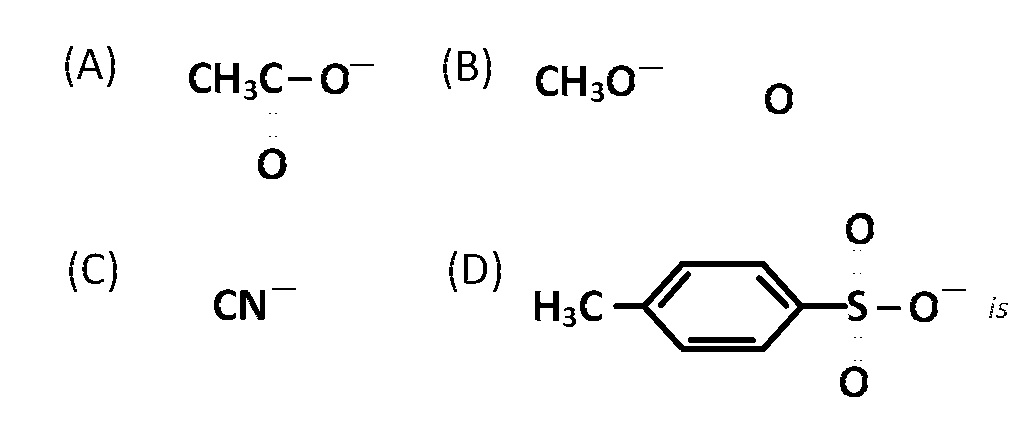

$$\left( C \right),\left( B \right),\left( A \right),\left( D \right)$$
$$\left( B \right),\left( C \right),\left( A \right),\left( D \right)$$
$$\left( D \right),\left( C \right),\left( B \right),\left( A \right)$$
$$\left( A \right),\left( B \right),\left( C \right),\left( D \right)$$
Explanation
Strong bases are generally good nucleophile. If the nucleophilic atom or the centre is the same, nucleophilicity parallels basicity, i.e., more basic the species, stronger is the nucleophile. Hence basicity as well as nucleophilicity order is
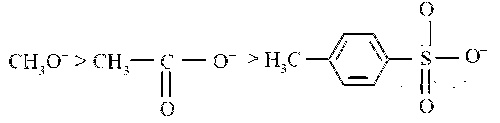
Now CN– is a better nucleophile than CH3O–. Hence decreasing order of nucleophilicity is
$$\left( C \right)>\left( B \right)>\left( A \right)>\left( D \right)$$
Now CN– is a better nucleophile than CH3O–. Hence decreasing order of nucleophilicity is
$$\left( C \right)>\left( B \right)>\left( A \right)>\left( D \right)$$
Comments (0)


