JEE MAIN - Chemistry (2005 - No. 23)
An organic compound having molecular mass 60 is found to contain C = 20%, H =
6.67% and N = 46.67% while rest is oxygen. On heating it gives NH3 along with a
solid residue. The solid residue give violet colour with alkaline copper sulphate
solution. The compound is
CH3NCO
CH3CONH2
(NH2)2CO
CH3CH2CONH2
Explanation
| Elements | % | Relative number of atoms | Simple Ratio |
|---|---|---|---|
| C | 20% | 20/12=1.66 | 1.66/1.66=1 |
| H | 6.67% | 6.67/1=6.67 | 6.67/1.66=4.16 |
| N | 46.67% | 46.67/14=3.33 | 3.33/1.66=2.02 |
| O | 26.64% | 26.64/16=1.66 | 1.66/1.66=1.0 |
The compound is $$C{H_4}{N_2}O$$
Empirical weight $$=60;$$ Mol. wt. $$=60;$$
$$\therefore$$ $$\,\,\,\,$$ $$n = {{60} \over {60}} = 1$$
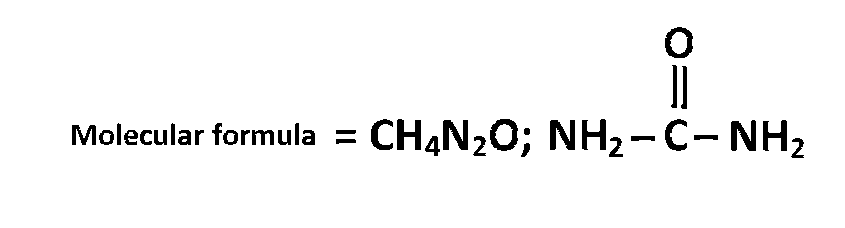
On heating urea losses ammonia to give Biuret
Biuret with alkaline $$CuS{O_4}$$ gives violet colour. Test for $$-CONH-$$group.
Comments (0)


