JEE MAIN - Chemistry (2004 - No. 9)
Consider the acidity of the carboxylic acids:
(a) PhCOOH
(b) o – NO2C6H4COOH
(c) p – NO2C6H4COOH
(d) m – NO2C6H4COOH
Which of the following order is correct?
(a) PhCOOH
(b) o – NO2C6H4COOH
(c) p – NO2C6H4COOH
(d) m – NO2C6H4COOH
Which of the following order is correct?
(b) > (d) > (a) > (c)
(b) > (d) > (c) > (a)
(a) > (b) > (c) > (d)
(b) > (c) > (d) > (a)
Explanation
In aromatic acids presence of electron withdrawing substituent e.g. $$ - N{O_2}$$ disperses the negative charge of the anion and stablises it and hence increases the acidity of the parent benzoic acid.
Further $$O$$- isomer will have higher acidity than corresponding $$m$$ and $$p$$ isomers. Since nitro group at $$p$$-position have more pronounced electron withdrawing than $$ - N{O_2}$$ group at $$m$$-position hence the correct order is the one given above.
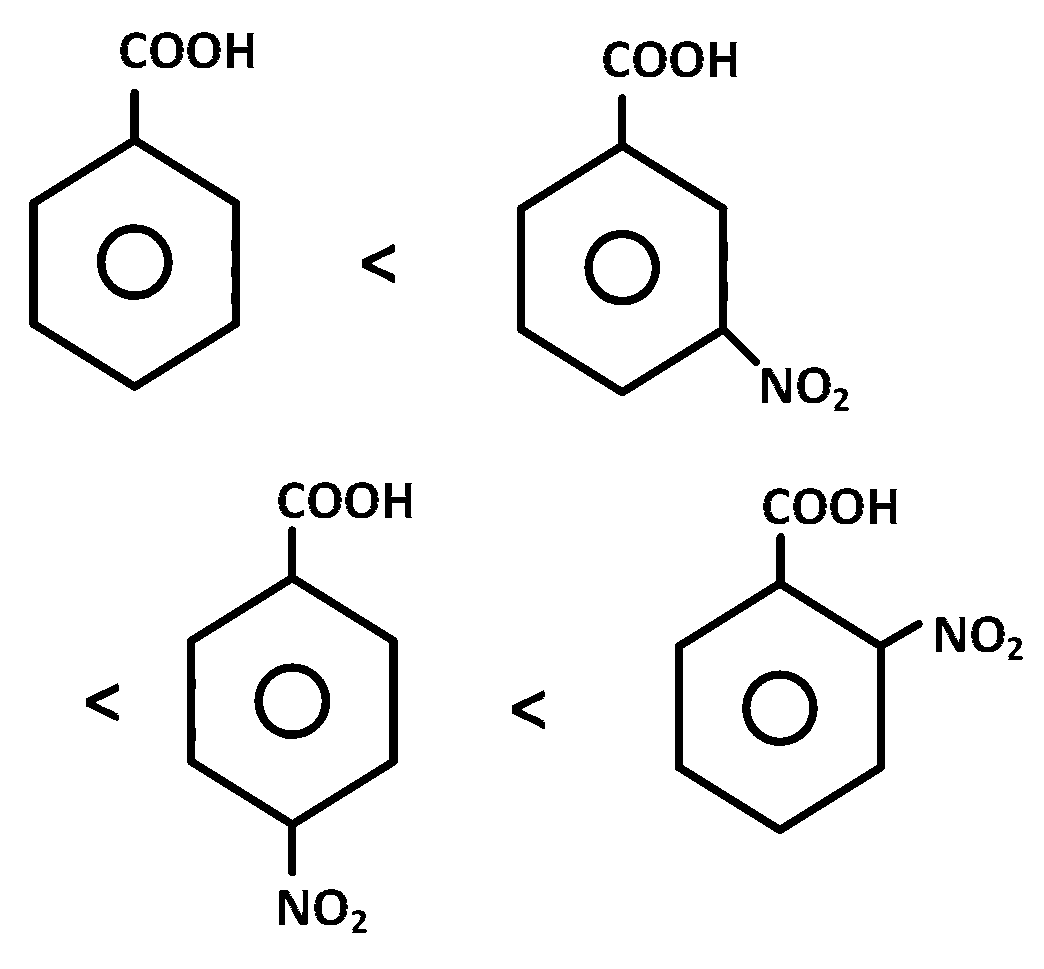
Further $$O$$- isomer will have higher acidity than corresponding $$m$$ and $$p$$ isomers. Since nitro group at $$p$$-position have more pronounced electron withdrawing than $$ - N{O_2}$$ group at $$m$$-position hence the correct order is the one given above.

Comments (0)


