JEE MAIN - Chemistry (2004 - No. 66)
The maximum number of 90° angles between bond pair of electrons is observed in
dsp3 hybridization
sp3d2 hybridization
dsp2 hybridization
sp3d hybridization
Explanation
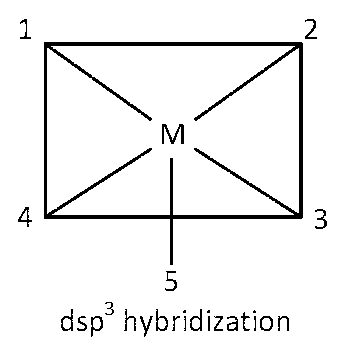
Here eight 90o angles between bond pair and bond pair. Those angles are $$\angle $$1M2, $$\angle $$2M3, $$\angle $$3M4, $$\angle $$4M1, $$\angle $$5M1, $$\angle $$5M2, $$\angle $$5M3, $$\angle $$5M4.
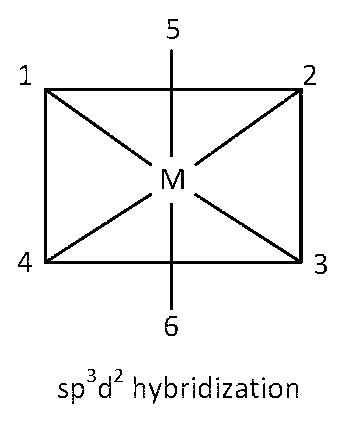
Here twelve 90o angles between bond pair and bond pair. Those angles are $$\angle $$1M2, $$\angle $$2M3, $$\angle $$3M4, $$\angle $$4M1, $$\angle $$5M1, $$\angle $$5M2, $$\angle $$5M3, $$\angle $$5M4, $$\angle $$6M1, $$\angle $$6M2, $$\angle $$6M3, $$\angle $$6M4.
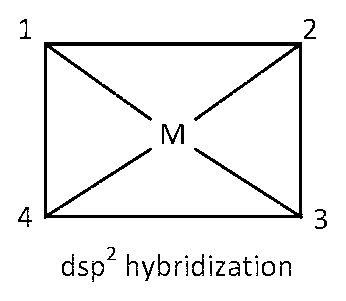
Here four 90o angles between bond pair and bond pair. Those angles are $$\angle $$1M2, $$\angle $$2M3, $$\angle $$3M4, $$\angle $$4M1.
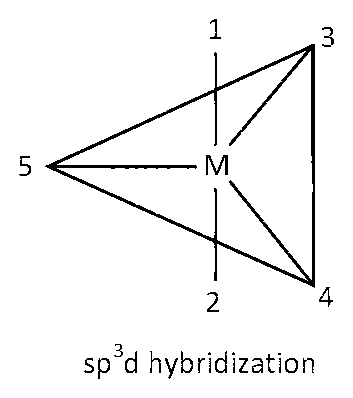
Here six 90o angles between bond pair and bond pair. Those angles are $$\angle $$1M3, $$\angle $$1M4, $$\angle $$1M5, $$\angle $$2M3, $$\angle $$2M4, $$\angle $$2M5.
Comments (0)


