JEE MAIN - Chemistry (2004 - No. 65)
Which one of the following has the regular tetrahedral structure?
(Atomic nos : B = 5, S = 16, Ni = 28, Xe = 54)
(Atomic nos : B = 5, S = 16, Ni = 28, Xe = 54)
XeF4
[Ni(CN)4]2-
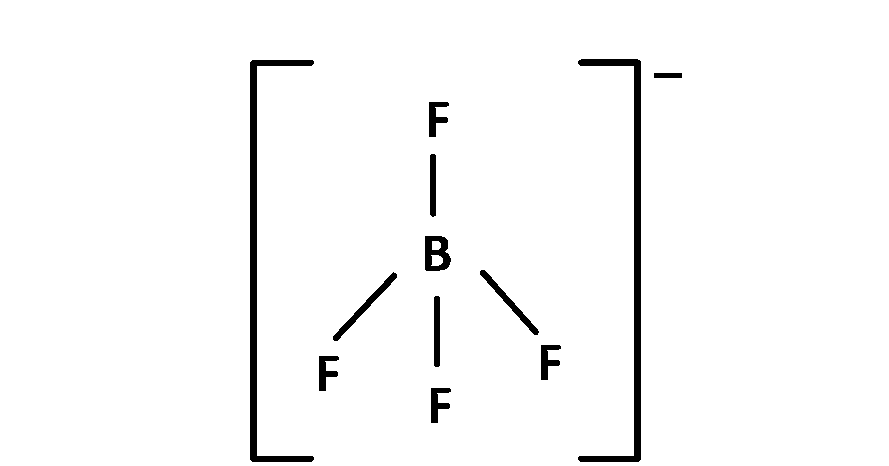
$$BF_4^-$$
SF4
Explanation
Regular Tetrahedral structure is possible in sp3 hybridization where central atom has 4 bond pair and no lone pair.
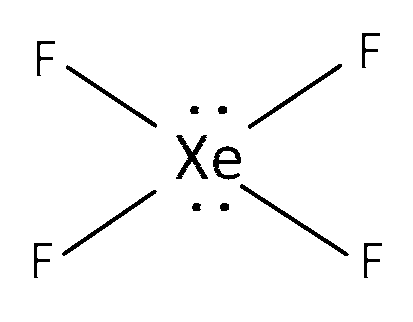
(a) XeF4 is sp3d2 hybridised and structure is square planar.
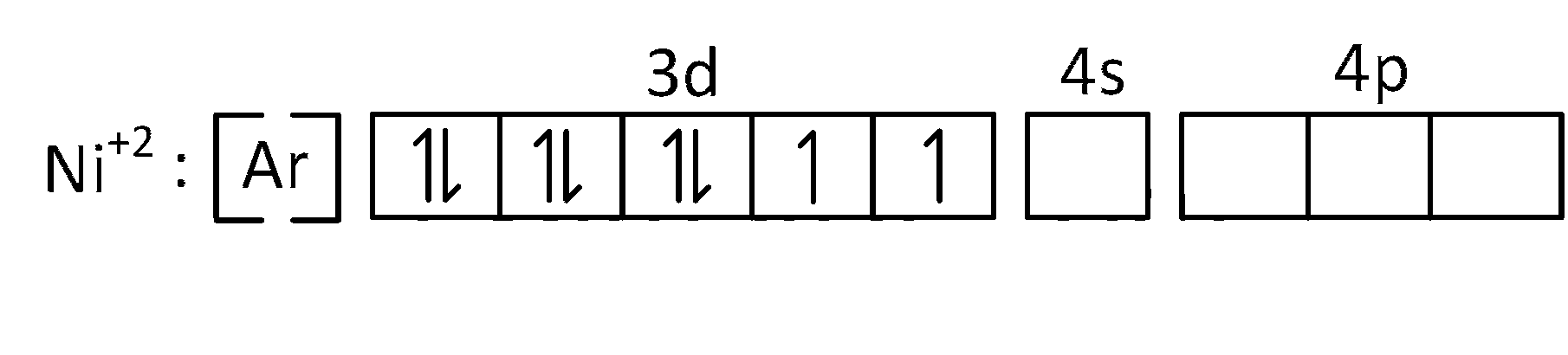
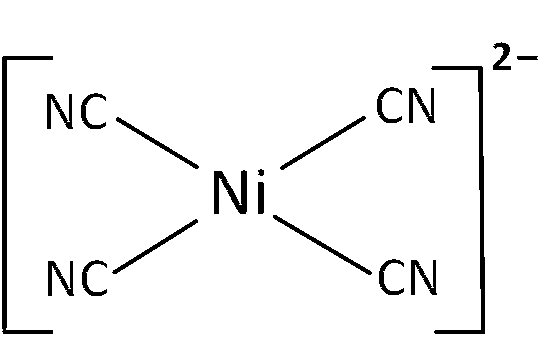
(b) [Ni(CN)4]$$-$$2 is coordinate compound and oxidation number of Ni is +2.
Electronic configuration of Ni+2 is $$=$$ [Ar]3d8
But because of CN$$-$$ ion which is a strong field ligand , it can perform pairing of electron.
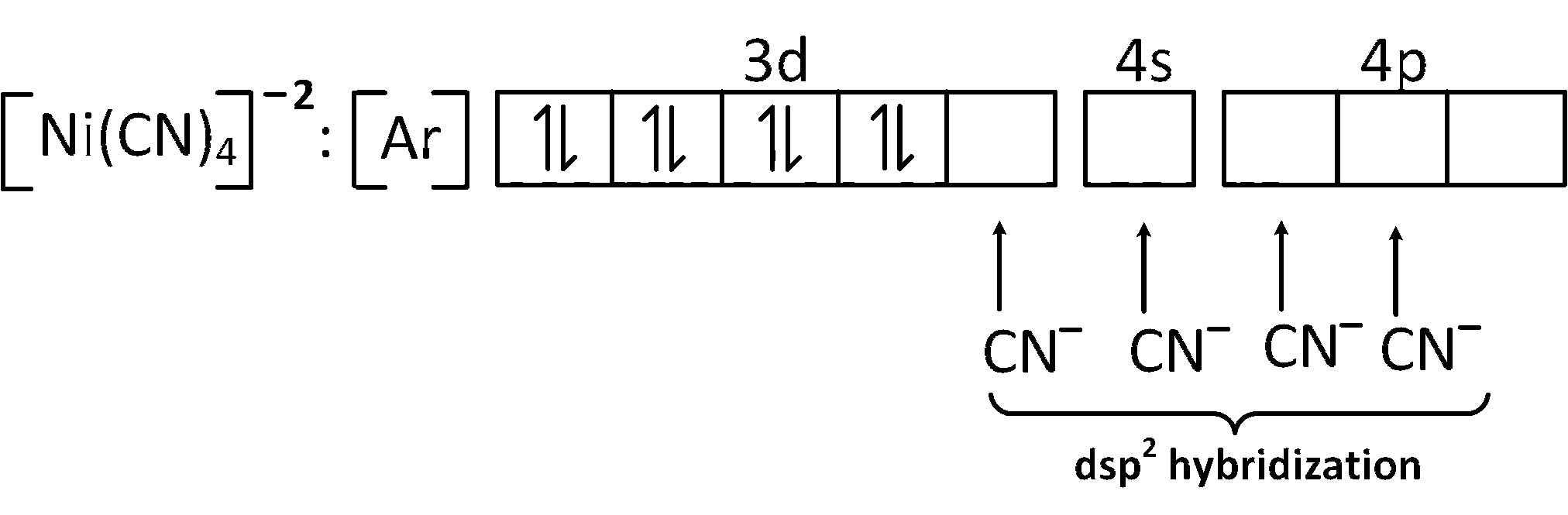
And the structure of dsp2 hybridization is square planar.
(C) $$\,\,\,\,$$ BF$$_4^ - $$, 4 bond pair present so angle is 109o 28' and sp3 hybridised. So structure is regular tetrahedral.
(d)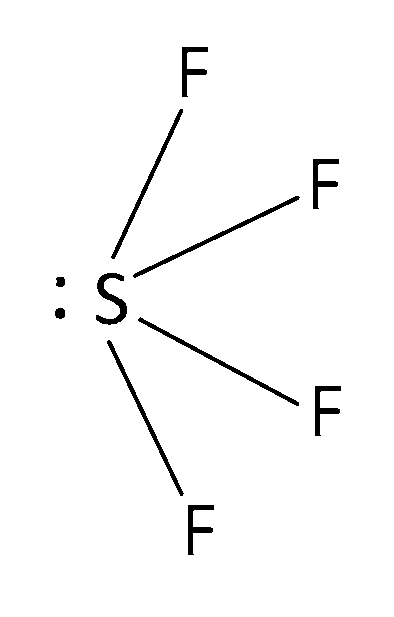
SF4 is sp3d hybridised and structure is see-saw.
(a) XeF4 is sp3d2 hybridised and structure is square planar.

(b) [Ni(CN)4]$$-$$2 is coordinate compound and oxidation number of Ni is +2.
Electronic configuration of Ni+2 is $$=$$ [Ar]3d8

But because of CN$$-$$ ion which is a strong field ligand , it can perform pairing of electron.

And the structure of dsp2 hybridization is square planar.

(C) $$\,\,\,\,$$ BF$$_4^ - $$, 4 bond pair present so angle is 109o 28' and sp3 hybridised. So structure is regular tetrahedral.

(d)

SF4 is sp3d hybridised and structure is see-saw.
Comments (0)


