JEE MAIN - Chemistry (2004 - No. 64)
The states of hybridization of boron and oxygen atoms in boric acid (H3BO3) are respectively
sp2 and sp2
sp3 and sp3
sp3 and sp2
sp2 and sp3
Explanation
Structure of H3BO3 is
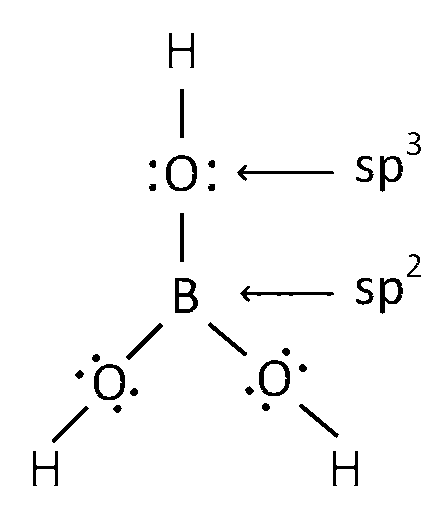
Here Boron has 3 sigma bond. So it is sp2 hybridised.
And oxygen has two sigma bond and two lone pair. So it sp3 hybridised.

Here Boron has 3 sigma bond. So it is sp2 hybridised.
And oxygen has two sigma bond and two lone pair. So it sp3 hybridised.
Comments (0)


