JEE MAIN - Chemistry (2004 - No. 31)
Which of the following liquid pairs shows a positive deviation from Raoult’s law?
Water – hydrochloric acid
Acetone – chloroform
Water – nitric acid
Benzene – methanol
Explanation
NOTE : Positive deviations are shown by such solutions in which solvent-solvent and solute-solute interactions are stronger than the solvent interactions. In such solution, the interactions among molecules becomes weaker. Therefore their escaping tendency increases which results in the increase in their partial vapour pressures.
In a solutions of benzene and methanol there exists inter molecular $$H-$$ bonding.
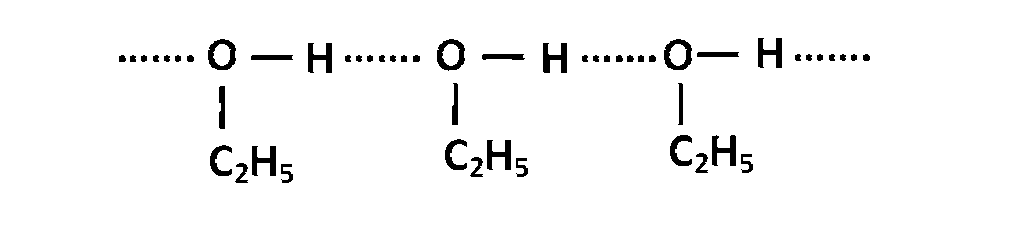
In this solution benzene molecules come between ethanol molecules which weaken intermolecular forces. This results in increase in vapour pressure.
In a solutions of benzene and methanol there exists inter molecular $$H-$$ bonding.

In this solution benzene molecules come between ethanol molecules which weaken intermolecular forces. This results in increase in vapour pressure.
Comments (0)


