JEE MAIN - Chemistry (2003 - No. 64)
Which one of the following compounds has the smallest bond angle in its molecule?
OH2
SH2
NH3
SO2
Explanation
(a) H2O is sp3 hybridized, and oxygen atom has 2 bond pair and 2 lone pair. So the angle between two O $$-$$ H bond is 104.5o
(b) In H2S molecule, central atom S is a 3rd period element and according to Dragos rule, when a 3rd period or higher period element overlap with a small element whose electronegetivity is low then that over lapping cannot be a effective overlapping, because of higher size of 3rd or higher periods element and smaller size of low electronegative element.
Here in H2S, H atom has very low electronegativity and smaller in size so it create more negative charge around sulphur(s) atom and size of S$$-$$2 ion increases, because of this higher size difference efficiency overlapping is not possible. So, any hybridization do not happen in between S and H atom. Lone pair electrons of S atom is present in the pure orbital like s, px, py, pz and it look like this :
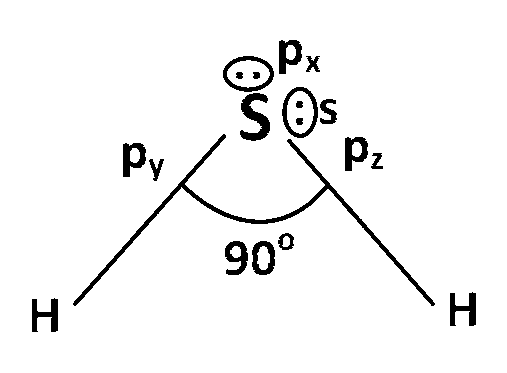
As we know the angle between and py is 90o, so bond angle is 90o.
(c) NH3 has sp3 hybridization and N atom has 3 bond pair and one lone pair so bond angle is 107o
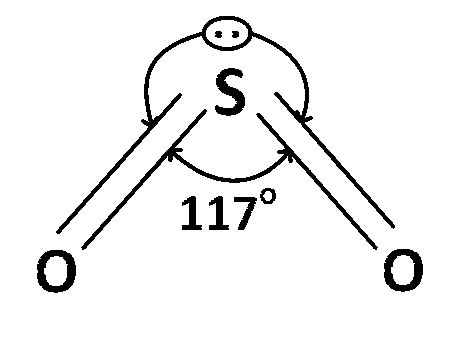
(d) SO2 is sp2 hybridized and bond angle is 117o.
(b) In H2S molecule, central atom S is a 3rd period element and according to Dragos rule, when a 3rd period or higher period element overlap with a small element whose electronegetivity is low then that over lapping cannot be a effective overlapping, because of higher size of 3rd or higher periods element and smaller size of low electronegative element.
Here in H2S, H atom has very low electronegativity and smaller in size so it create more negative charge around sulphur(s) atom and size of S$$-$$2 ion increases, because of this higher size difference efficiency overlapping is not possible. So, any hybridization do not happen in between S and H atom. Lone pair electrons of S atom is present in the pure orbital like s, px, py, pz and it look like this :

As we know the angle between and py is 90o, so bond angle is 90o.
(c) NH3 has sp3 hybridization and N atom has 3 bond pair and one lone pair so bond angle is 107o
(d) SO2 is sp2 hybridized and bond angle is 117o.

Comments (0)


