JEE MAIN - Chemistry (2003 - No. 63)
Which one of the following pairs of molecules will have permanent dipole moments for both members
NO2 and CO2
NO2 and O3
SiF4 abd CO2
SiF4 abd NO2
Explanation
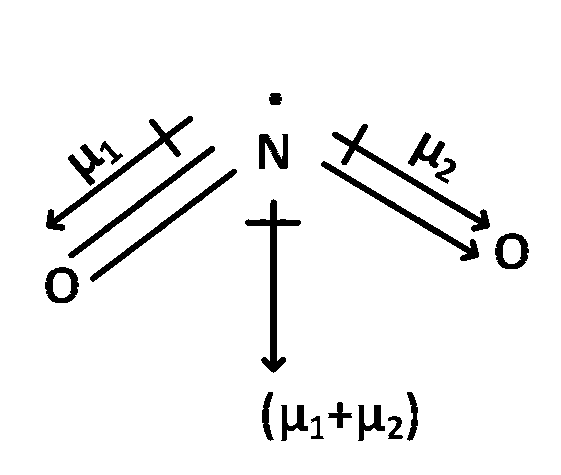
Here $$\mu $$total = $$\mu $$1 + $$\mu $$2 $$ \ne $$ 0
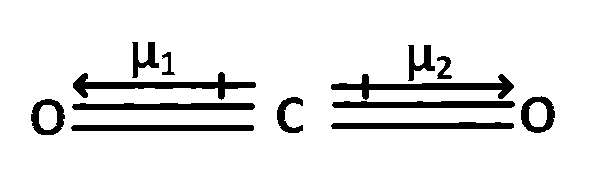
$$\mu $$total = $$\mu $$1 $$-$$ $$\mu $$2 = 0
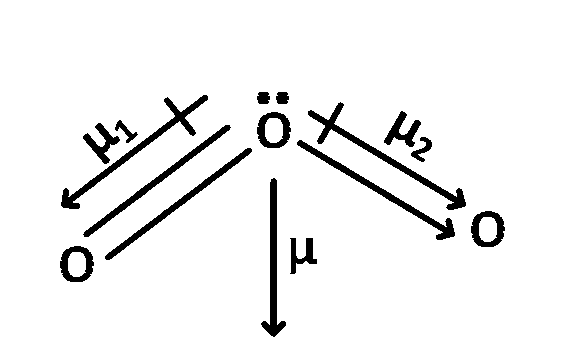
$$\mu $$total = $$\mu $$1 + $$\mu $$2 $$ \ne $$ 0
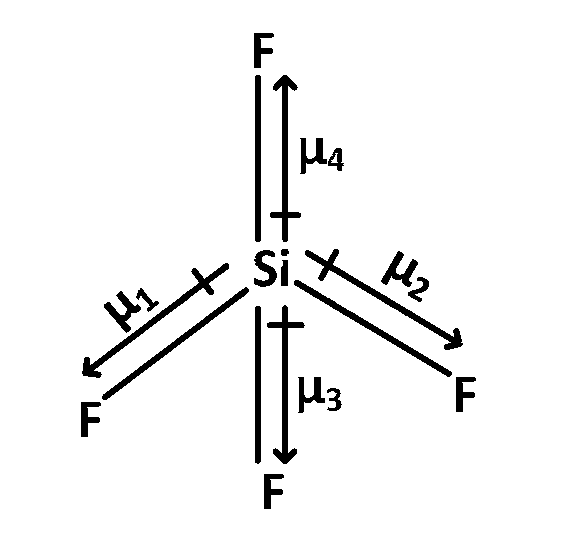
$$\mu $$1 + $$\mu $$2 + $$\mu $$3 = $$\mu $$4
$$\mu $$total = 0
In NO2 and O3 have permanent dipole moment as both of them are not symmetric molecule.
In CO2 and SiF4 each individual bond c = O and S- $$-$$ F have dipole moment but because of symmetric structure individual dipole moment get's cancelled.
Comments (0)


