JEE MAIN - Chemistry (2003 - No. 62)
An ethar is more volatile than an alcohol having the same molecular formula. This is due to
alcohols having resonance structures
inter-molecular hydrogen bonding in ethers
inter-molecular hydrogen bonding in alcohols
dipole characters of ethers
Explanation
Alcohol and ether are isomer with each other. So, with same molecular formula we can make ether as well as alcohol.
For ex,
With molecular formula C2H6O
(1) $$\,\,\,$$ alcohol will be CH3CH2 OH
(2) $$\,\,\,$$ ether will be CH3 $$-$$ O $$-$$ CH3
In Alcohol there is hydrogen bond and in Ether there is Van der walls force of attraction.
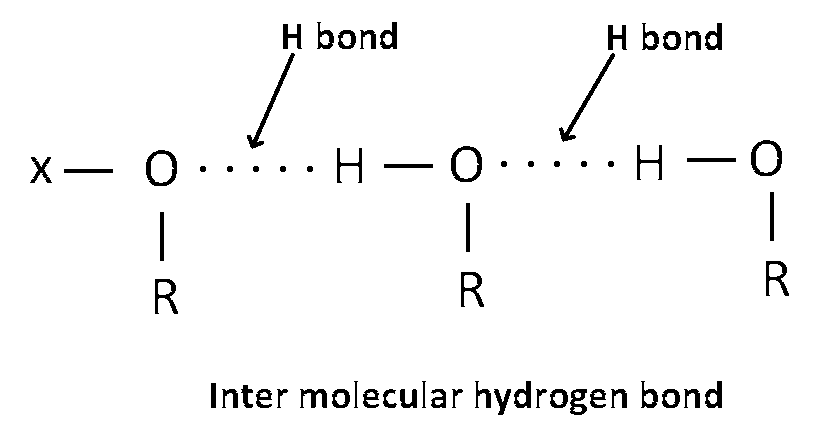
We know that H bond is stronger bond than van der walls force of attraction as the atoms of alcohol are strongly attached with each other by hydrogen bonding so tendency of vaporization of alcohol is less compared to ether.
In alcohol inter-molecular hydrogen bonding look like this -
For ex,
With molecular formula C2H6O
(1) $$\,\,\,$$ alcohol will be CH3CH2 OH
(2) $$\,\,\,$$ ether will be CH3 $$-$$ O $$-$$ CH3
In Alcohol there is hydrogen bond and in Ether there is Van der walls force of attraction.
We know that H bond is stronger bond than van der walls force of attraction as the atoms of alcohol are strongly attached with each other by hydrogen bonding so tendency of vaporization of alcohol is less compared to ether.
In alcohol inter-molecular hydrogen bonding look like this -

Comments (0)


