JEE MAIN - Chemistry (2003 - No. 58)
In Bohr series of lines of hydrogen spectrum, the third line from the red end corresponds to which one of the following inter-orbit jumps of the electron for Bohr orbits in an atom of hydrogen
5 $$\to$$ 2
4 $$\to$$ 1
2 $$\to$$ 5
3 $$\to$$ 2
Explanation
In Bohr series of lines of hydrogen spectrum :-
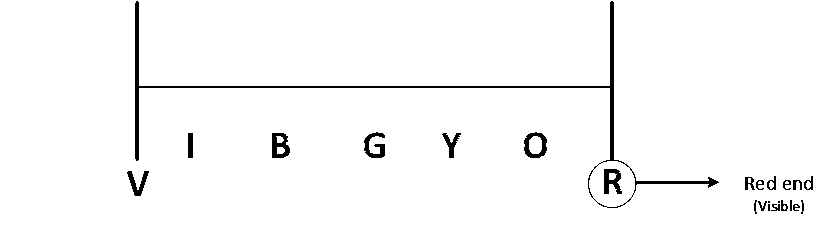 Red end means end of visible region which is balmer series of lines so n will be 2
Red end means end of visible region which is balmer series of lines so n will be 2
Now for third lines with respect to Balmer series means n = (2 + 3) = 5th line
$$\therefore$$ n1 = 2 and n2 = 5
For 2 $$\to$$ 5 electron will need energy to jump as 2 > 5 so no spectrum will release instead energy will be absorbed.
$$\therefore$$ 5 $$\to$$ 2
 Red end means end of visible region which is balmer series of lines so n will be 2
Red end means end of visible region which is balmer series of lines so n will be 2Now for third lines with respect to Balmer series means n = (2 + 3) = 5th line
$$\therefore$$ n1 = 2 and n2 = 5
For 2 $$\to$$ 5 electron will need energy to jump as 2 > 5 so no spectrum will release instead energy will be absorbed.
$$\therefore$$ 5 $$\to$$ 2
Comments (0)


