JEE MAIN - Chemistry (2003 - No. 48)
Ammonia forms the complex ion [Cu(NH3)4]2+ with copper ions in alkaline solutions but not in acidic solutions. What is the reason for it?
In acidic solutions protons coordinate with ammonia molecules forming $$NH^+_4$$ ions and NH3
molecules are not available
In alkaline solutions insoluble Cu(OH)2
is precipitated which is soluble in excess of any alkali
Copper hydroxide is an amphoteric substance
In acidic solutions hydration protects copper ions.
Explanation
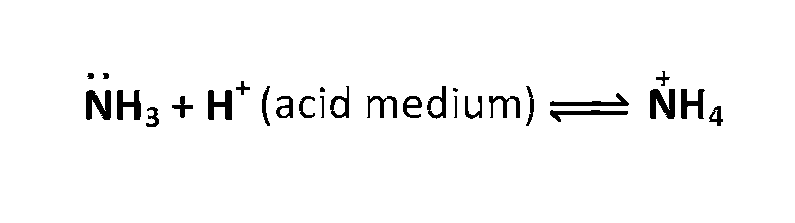
Comments (0)


