JEE MAIN - Chemistry (2003 - No. 2)
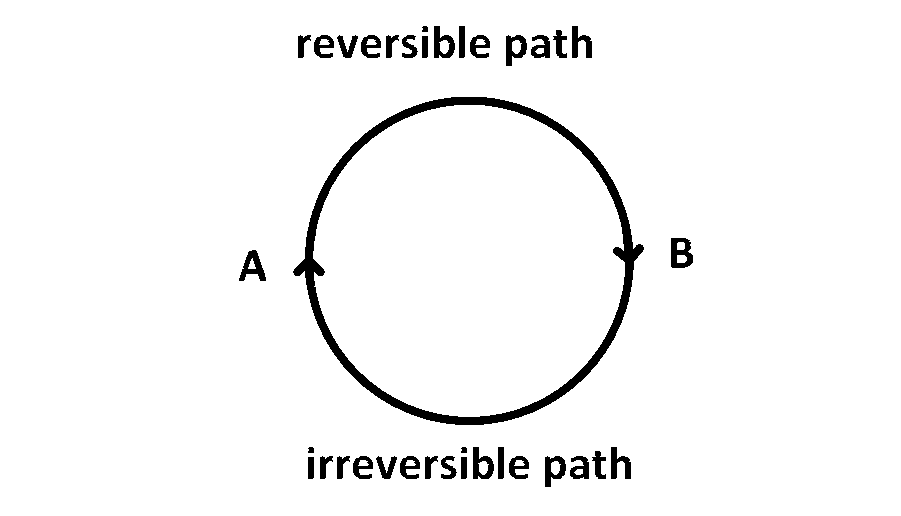
The internal energy change when a system goes from state A to B is 40 kJ/mole. If the system goes from A to B by a reversible path and returns to state A by an irreversible path what would be the net change in internal energy?
> 40 kJ
< 40 kJ
Zero
40 kJ
Explanation
For a cyclic process the net change in the internal energy is zero because the change in internal energy does not depend on the path.

Comments (0)


