JEE MAIN - Chemistry (2003 - No. 12)
In the anion HCOO$$-$$
the two carbon-oxygen bonds are found to be of equal length. What is the reason for it?
The C = O bond is weaker than the C-O bond
The anion HCOO-
has two resonating structures
The anion is obtained by removal of a proton from the acid molecule
Electronic orbitals of carbon atom are hybridised
Explanation
The anion is
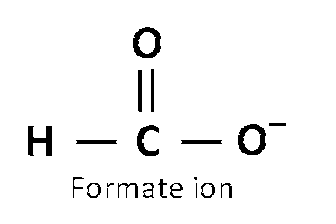
$$HCO{O^ - }$$ exists in following resonating structures
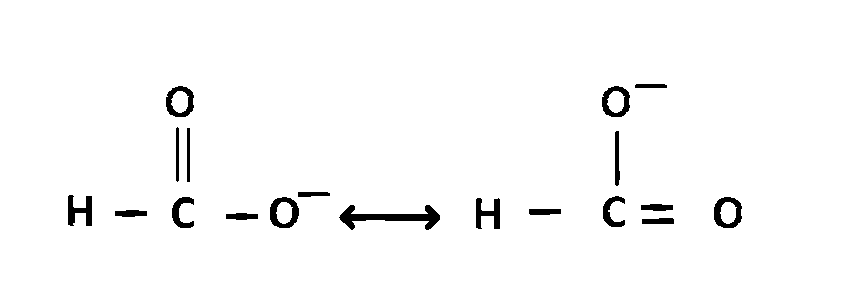
And actual structure will be,
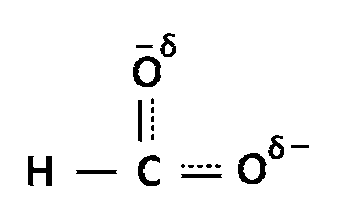
Bond order of this anion = $${3 \over 2}$$ = 1.5.
As 1.5 is exactly in the middle of 1 and 2 so, the bond length will be equal between all carbon and oxygen atom.
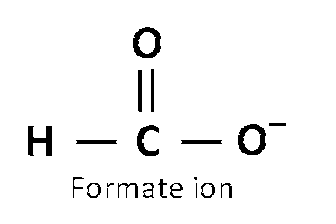
$$HCO{O^ - }$$ exists in following resonating structures

And actual structure will be,
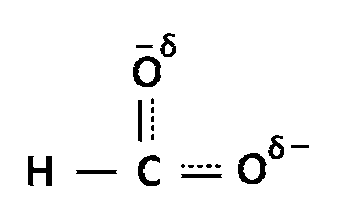
Bond order of this anion = $${3 \over 2}$$ = 1.5.
As 1.5 is exactly in the middle of 1 and 2 so, the bond length will be equal between all carbon and oxygen atom.
Comments (0)


