JAMB - Chemistry (2024 - No. 28)
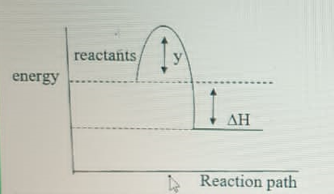
In the graph above, y represents
endothermic reaction
activation energy
exothermic reaction
ionization energy
Explanation
The activation energy is the energy required to get the reaction going. It's the difference between the energy of the reactants and the peak of the hump.
Comments (0)


