JAMB - Chemistry (2018 - No. 55)
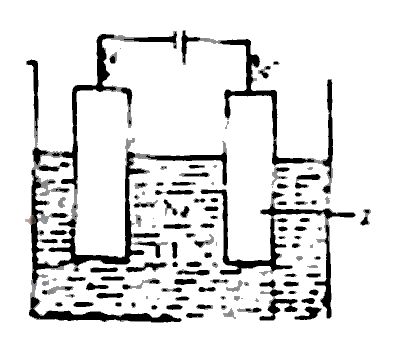
The figure above shows the electrolysis of molten sodium chloride. Z is the
anode where the Cl\(^-\) ions are oxidized
cathode where the Cl\(^-\) ions are reduced
anode where the Na\(^+\) ions are reduced
cathode where the Na\(^+\) ions are oxidized
Explanation
During the electrolysis of brine, the chlorine ions are discharged at the anode and are oxidized. While at the cathode Hydrogen ion is discharged.
Comments (0)


