JAMB - Chemistry (2013 - No. 35)
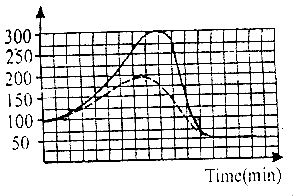
In the graph above, the activation energy of the catalyzed reaction is
100KJ
300KJ
250KJ
200KJ
Explanation
In the graph above, the activation energy of the catalyzed reaction is shown by the curve with the broken lines. Tracing it to the peak of the hump and extrapolating, we have 200kJ. However, to find the activation energy, it is the difference between the energy of the peak of the hump and energy of the reactant.
i.e Activation energy = Energy at the peak of the hump - energy of the reactant
= 200 - 100
= 100kJ
Comments (0)


